The AITD, including Hashimoto’s thyroiditis and Graves’ disease, are prevalent conditions that affect the thyroid gland’s function due to development of anti-thyroid autoantibodies. The importance of selenium in managing these AITDs has garnered significant attention in medical research and clinical practice [1].
Selenium is a trace element essential for normal health, playing a crucial role in thyroid hormone synthesis and metabolism. The thyroid gland contains a high concentration of selenium, particularly in the form of selenoproteins such as Glutathione Peroxidases (GPX) and thioredoxin reductases [2]. These selenoproteins help protect thyroid cells from oxidative damage and regulate thyroid hormone production [3].
In AITDs, selenium has been found to have several important functions, including antioxidant defense, modulation of immune response and thyroid hormone regulation. Selenium is present in the GPX, which nullifies the oxidative damage of hydrogen peroxide during synthesis of thyroid hormones [4].
Selenium acts as a potent antioxidant, counteracting the oxidative stress that occurs during autoimmune attacks on the thyroid gland [5,6]. By neutralising harmful free radicals, selenium helps reduce inflammation and protect thyroid tissue from damage. Selenium has immunomodulatory effects, influencing the activity of immune cells and cytokines involved in autoimmune reactions [7]. It helps regulate the balance between pro-inflammatory and anti-inflammatory pathways, potentially mitigating the autoimmune process in the thyroid gland. Selenium is necessary for the conversion of thyroxine (T4) to triiodothyronine (T3), the active form of thyroid hormone [1]. In AITDs, maintaining optimal selenium levels may improve thyroid hormone levels and alleviate symptoms such as fatigue, weight changes and mood disturbances [1].
Estimating selenium levels in AITD serves a critical rationale due to selenium’s pivotal role in thyroid function. Selenium is integral for thyroid hormone synthesis, protecting against oxidative stress and modulating of immune responses [3]. In AITD, selenium deficiency can exacerbate inflammation, impair thyroid hormone production and escalate tissue damage. Monitoring selenium levels aids in tailoring effective interventions, such as selenium supplementation, to mitigate autoimmune responses, enhance thyroid functions and improve patient outcomes [6].
A meta-analysis by Wichman J et al., underscores the rationale for estimating selenium in AITD [8]. This analysis, which included 16 randomised controlled trials, demonstrated that selenium supplementation significantly reduced Thyroid Peroxidase Antibodies (TPOAb) and Thyroglobulin Antibodies (TgAb) levels in patients with Hashimoto’s thyroiditis. These findings highlight the potential of selenium to modulate autoimmune responses and improve thyroid function in such disorders. Monitoring selenium levels and considering supplementation can thus be crucial in managing autoimmune thyroid conditions effectively.
Another meta-analysis involving 10 studies (with 796 subjects) by Zheng H et al., further supports the rationale of supplementing the selenium in AITDs [9]. This analysis included studies evaluating selenium supplementation in patients with Grave’s disease and found that selenium supplementation led to a significant reduction in free T4 levels. In addition, there was decrease in thyrotrophic hormone receptor antibody at six months post-supplementation [9].
Despite the established role of selenium in thyroid function and its potential therapeutic benefits in AITD, significant gaps remain in understanding the precise relationship between selenium levels and the risk of developing AITD in specific populations. Most existing studies have focused on the impact of selenium supplementation rather than directly investigating baseline selenium levels and their association with autoimmune markers and disease severity [5,6]. Furthermore, the available meta-analyses primarily analyse Western populations [8,9], leaving a critical gap in data from diverse geographical regions, particularly from selenium-deficient or selenium-excess regions like parts of Asia. This study addresses these gaps by evaluating serum selenium levels and their correlation with thyroid autoantibodies in AITD patients in an Indian population. The novelty of the present study lies in its population-specific focus and its attempt to quantify the risk of AITD in individuals with low selenium levels, providing insights for tailored interventions in similar resource-constrained settings.
With this background, it is evident that assessing the selenium status as part of comprehensive management strategies for AITDs is pivotal. Therefore, the objective of the study was to evaluate the selenium levels and autoantibody levels in AITD and to establish the risk of AITD among patients with low selenium levels.
Materials and Methods
The present case-control study was conducted in the Department of Surgery, Department of Medicine, Department of Biochemistry and Department of Physiology at MOSC Medical College, Kolenchery, Kerala, India from July 2022 to May 2023. The study was approved by the Institutional Ethics Committee (IEC letter number: MOSC/IEC/645/2022 dated 01-07-2022). An informed consent process was undertaken for all participants and confidentiality and privacy of their data were ensured.
Inclusion criteria for cases: Patients who had signs and symptoms of thyroid disorders and confirmed with laboratory results meeting following inclusion criteria were selected as cases. The study utilised institutional reference ranges for thyroid hormones, defined as T4: 5.5-11 μg/dL, T3: 0.97-1.69 ng/mL and TSH: 0.46-5 mIU/mL, to interpret thyroid function test results. Age range of patients was between 20 and 60 years. Patients must have a confirmed diagnosis of an AITD, such as Hashimoto’s thyroiditis or Graves’ disease, based on at least one of the thyroid function tests: TSH above the upper limit of reference range (>5.0 mIU/L), below the lower limit of the reference range (<0.46 mIU/L), T4 below the lower limit of reference range (<5.5 μg/dL), above the upper limit of reference range (>11 μg/dL), T3 below the lower limit of reference range (<0.97 ng/mL), or above the upper limit of reference range (>1.69 ng/mL). In addition, thyroid autoantibodies (institutional reference ranges) were evaluated. Anti-TPO more than 50 IU/L is considered as positive result, or Anti-TGO antibody more than 125 IU/L is considered as positive result. Histopathological examination (if available) confirming the diagnosis of either Hashimoto’s thyroiditis or Grave’s disease were included.
Exclusion criteria for cases: Individuals with non AITDs (e.g., thyroid nodules, thyroid cancer) or thyroid dysfunction due to non autoimmune causes (e.g., iodine deficiency, medication-induced thyroid dysfunction) were excluded. Individuals who are currently taking thyroid hormone replacement therapy or other medications that can affect thyroid function, as these can confound the relationship between selenium levels and thyroid disorders, were excluded. Pregnant women, as pregnancy can significantly impact thyroid function and selenium levels were excluded. Subjects who have underwent thyroidectomy subjects or any reason were excluded. Additionally, individuals with chronic illnesses or conditions that can affect selenium metabolism or thyroid function independently of AITDs and those on treatment with lithium, amiodarone and other drugs which affect thyroid function, were excluded.
Matching criteria: Age and gender-matched controls were selected. Such critical matching negated potential confounders and ensured that any observed differences in selenium levels between cases and controls are more likely due to AITD status rather than demographic variations.
Inclusion criteria for controls: Objective of control selection was to match controls to cases based on demographic factors that may influence selenium levels and the risk of AITD.
Exclusion criteria for controls: Individuals with any thyroid disorder or other significant health conditions that could affect selenium levels independently of AITD were strictly excluded.
General exclusion criteria for both AITD and control subjects: Excessive consumption of selenium-rich foods, like yellowfin tuna, sardines, shrimp and Brazil nuts, or selenium supplements (e.g., selenised yeast) can lead to elevated selenium levels, potentially reaching toxic levels. Therefore, subjects with known history of selenium supplement in any form, including selenium methionine (present in dietary supplements and multivitamins medications), selenium selenite, selenium selenate (both present in selenium supplements and fortified foods), selenomethionine yeast (commonly used in selenium supplements), were excluded from both AITD subjects and control groups.
Sample size calculation: Sample size calculation when comparing proportions between cases and control groups was based on estimating the OR. The formula takes into account the expected proportion exposed in the control group (P2), the estimated OR, the significance level (α) and the desired power (1-β). The formula is as follows:
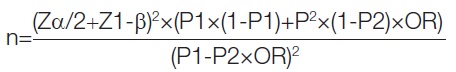
where n is the required sample size per group (cases or controls), Z score corresponds to 95% confidence limit, P1 calculated proportion in the case group, P2 expected proportion exposed in the control group, OR estimated OR, α is minimum number of required discordant pairs and 1-β is the power.
Sample size was calculated from the reference study by Wu Q et al., for the case-control study with equal allocation (1:1) [10]. They studied 6,152 participants and found proportion of autoimmune thyroiditis of 24.4%. The OR of autoimmune thyroiditis in the high serum selenium group is 0.35. Therefore, two-sided sample size for power 80% and an alpha error of 5% was calculated using the above formula, arriving at a total of 67 for cases and controls.
Study Procedure
A predesigned and pretested questionnaire, which including nutritional history and family history, was distributed among cases and controls to collect the demographic details. Through this age, sex, regionality, previous medical and surgical history, family history of thyroid disorders or any other autoimmune disorders and history of nutritional supplementation was collected.
After signing informed consent document, height and weight details were collected from all participants. A physical examination was undertaken where any thyroid enlargement, pallor, lymph node enlargement, head to toe examination for signs of hypothyroidism and hyperthyroidism.
Blood samples collected at 6-8 AM were used to estimating thyroid function status (T4, T3 and TSH). The presence of subclinical, hypo-, or hyperthyroidism were used to define thyroid dysfunction.
Participants with thyroid disorders were further evaluated for thyroid antibodies- anti-Thyroid Peroxidase (anti-TPO) and anti-TgAb (antiTGO) measured by Electro Chemiluminescence Immunoassay (ECLIA). Serum selenium was estimated using Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
Statistical Analysis
Categorical variables were summarised using frequency and percentage. Quantitative variables were summarised as mean and standard deviation. The relationship between thyroid hormones, antibodies and serum selenium and iodine levels were studied using Pearson’s correlation coefficient. The OR of AITD in patients with low selenium levels was calculated. The Chi-square test evaluated whether the observed association between AITD and selenium status is statistically significant, using formula, χ2=∑(Oi-Ei)2/Ei. Where Oi is the observed value (actual value) and Ei is the expected value. Assuming the null hypothesis (no association) and using a significance level (alpha) of 0.05, with one degree of freedom, Chi-square value was calculated and association between AITD and selenium status was considered.
Results
The study included 67 AITD patients, comprising 38 hypothyroid and 29 hyperthyroid subjects. Age ranged from 20 to 60 years. There was no statistical difference with respect to gender and age (p=0.11, 0.087, respectively) between AITD patients and euthyroid controls [Table/Fig-1].
Tabulation of baseline characteristics of cases (n, 67) and controls (n, 67).
| Demographic parameters | Hypothyroidism (n=38) | Hyperthyroidism (n=29) | Control (n=67) |
|---|
| Female | 28 | 7 | 35 |
| Male | 10 | 22 | 32 |
| Age (in years) | 42.6±14.7 | 38.4±16.1 |
| Positive family history | 27 | 14 | 0 |
Thyroid hormones, thyroid antibodies and relation with selenium: The average thyroid hormones, thyroid antibodies and selenium levels in controls and AITDs patients are tabulated in [Table/Fig-2]. Mean selenium levels among AITDs cases was 0.088±0.07 μg/mL and in controls they were 0.12±0.01 μg/mL. The difference was statistically significant, with p-values <0.01. The mean selenium levels in patients with hypothyroidism were 0.082±0.053 μg/mL and in patients with hyperthyroidism, they were 0.081±0.013 μg/mL. There were two subjects with low selenium levels among the euthyroid controls [Table/Fig-2]. From [Table/Fig-2], it is evident that selenium levels among hypothyroidism and hypothyroidism subjects were statistically lower in comparison to age- and sex-matched euthyroid controls.
Tabulation of thyroid hormones, thyroid antibodies and serum selenium among AITD and controls, values provided as mean±standard deviation.
| Subjects | Cases | Controls | p-value |
|---|
| Hypothyroidism | n=38 | n=67 | |
| T3 (in ng/mL) | 0.89±0.27 | 1.20±0.2 | <0.0001 |
| T4 (in μg/dL) | 4.81±2.26 | 6.84±2.44 | <0.0001 |
| TSH (in uIU/mL) | 19.77±31.9 | 2.10±1.04 | <0.001 |
| TPO (in IU/mL) | 405±366 | 30.1±5.2 | <0.0001 |
| TGO (in IU/mL) | 141±97 | 49.8±8.5 | <0.0001 |
| Selenium (μg/mL) | 0.08±0.05 | 0.11±0.01 | <0.001 |
| Hyperthyroidism | n=29 | n=67 | |
| T3 (in ng/mL) | 3.17±0.83 | 1.20±0.2 | <0.0001 |
| T4 (in μg/dL) | 11.19±2.6 | 6.84±2.44 | <0.0001 |
| TSH (in uIU/mL) | 0.2±0.2 | 2.10±1.04 | <0.0001 |
| TPO (in IU/mL) | 310±32.8 | 30.1±5.2 | <0.0001 |
| TGO (in IU/mL) | 115±10.5 | 49.8±8.5 | <0.0001 |
| Selenium (μg/mL) | 0.08±0.01 | 0.11±0.01 | <0.0001 |
Among cases, a positive Pearson correlation was observed between T3, T4, TPO, TGO levels and GPX selenium levels; however, the correlation was non significant. Correlation between TSH and serum selenium levels was negative in both hypo- and hyperthyroid cases. Among controls, a negative correlation was observed between T3, TSH, TPO and TGO levels with serum selenium, while TSH levels positively correlated with serum selenium values. However, these correlations were non significant [Table/Fig-3].
Tabulation of Pearson’s correlation depicting r-values serum selenium levels with other parameters evaluated.
| Pearson’s correlation | Size | T3 and selenium | T4 and selenium | TSH and selenium | TPO and selenium | TGO and selenium |
|---|
| Hypothyroidism | 38 | 0.094
(p=0.57) | 0.218
(p=0.19) | -0.083
(p=0.52) | 0.312
(p=0.10) | 0.042
(p=0.58) |
| Hyperthyroidism | 29 | 0.094
(p=0.57) | 0.349
(p=0.10) | -0.201
(p=0.17) | 0.063
(p=0.73) | 0.217
(p=0.19) |
| Controls | 67 | -0.018
(p=0.85) | 0.080
(p=0.52) | -0.175
(p=0.85) | -0.077
(p=0.51) | -0.120
(p=0.33) |
The OR quantifying the association between AITD and low selenium levels compared to normal selenium levels [Table/Fig-4] was calculated as 8.6 (95% CI 0.619-3.677), indicating that individuals with AITD are about 8.6 times more likely to have low selenium levels compared to those without AITD. This suggests a strong positive association between AITD and low selenium.
Contingency table showing the relationship between serum selenium levels with Autoimmune Thyroid Disorders (AITD) (AITD, n=67) and controls (n=67).
| Serum selenium | AITH | Controls | p-value | Odds Ratio (OR) | Relative Risk (RR) |
|---|
| Number of subjects with low selenium | 14 (20.9%) | 2 (2.99%) | 0.0034 | 8.6 | |
| Number of subjects with normal selenium | 53 (79.1%) | 65 (97.01%) | | 1.95 |
The RR calculated was 1.95, indicates that individuals with selenium deficiency are more likely to have AITD compared to those with normal selenium levels. This implies a moderate positive association between selenium deficiency and AITD risk. Attributable risk (prevalence of AITD cases with low selenium levels, 0.875 - prevalence of AITD cases with normal selenium levels, 0.125) was calculated as 0.75, indicating that 75% of AITD cases with low selenium levels can be attributed to the reduced selenium itself. In other words, selenium deficiency contributes significantly to the occurrence of AITD among affected individuals.
The Chi-square test value was 8.59 (p-value: 0.0034 and Phi coefficient - effect size: 0.253), indicating that there is a statistically significant association between AITD and selenium status at a certain level of significance [Table/Fig-4].
Discussion
The findings of the present study highlight a significant association between serum selenium levels and AITDs (p=0.0034) and an OR of 8.60. Selenium deficiency was markedly more prevalent in AITD cases compared to controls, with a RR of 1.95, underscoring the heightened likelihood of developing AITD in the context of low selenium levels. These results align with existing literature suggesting selenium’s critical role in thyroid function and immune regulation, as selenium is essential for the enzymatic activity of GPX and other selenoproteins that protect thyroid cells from oxidative damage [2,3,11]. The observed negative correlation between selenium levels and Thyroid Stimulating Hormone (TSH), particularly in hypothyroid and hyperthyroid subjects, further underscores selenium’s involvement in thyroid hormone metabolism and immune modulation. These data support the hypothesis that selenium supplementation may offer therapeutic benefits in managing AITD.
Some studies suggest that individuals with hypothyroidism may have an increased requirement for selenium due to changes in selenium metabolism and utilisation in thyroid hormone synthesis [5,6]. While observational studies suggest low selenium status as an iodine-independent risk factor for goiter [12], intervention trials in humans are lacking. In the present study, statistically significant lower selenium levels were noted in both hypothyroid (p<0.001) and hyperthyroid (p<0.0001) subjects in comparison to controls. Similar to the present study’s findings, Owji N et al., while evaluating the selenium levels in Graves’s disease patients with or without ophthalmopathy among a subset of 60 Egyptian patients, found a reverse association between serum selenium levels and thyroid hormones—especially T3 and T4 [13]. Furthermore, in their analysis, there was no statistical difference in serum selenium levels between subjects with Graves’s ophthalmopathy and those without ophthalmopathy. In a similar study by Hasanin GA et al., mean selenium levels were observed to be reduced with increasing severity of Graves’s disease [14]. In a case-control study comparing selenium status in AITDs in an iodine-sufficient area, Heidari Z and Sheikhi V found that selenium was deficient among 15.2%, 2.5% and 2.5% of Graves’s disease, Hashimoto’s thyroiditis and controls, respectively [15]. However, in the present study, there were two euthyroid control subjects with selenium deficiency.
In a cross-sectional observational study by Wu Q et al., the prevalence of thyroid dysfunction was significantly higher among people with low selenium levels. Higher selenium was associated with lower OR of (0.47; 05% CI 0.35-0.65) for autoimmune thyroid conditions [10]. In a study examining the association between thyroid hormone status and serum selenium in patients with nodular goiter, Kravchenko VI et al., observed a higher RR of goiter at lower serum selenium levels (OR=1.63, 95% CI: 1.16-1.78, p<0.05) in comparison with OR values in the control group [16]. Epidemiological data from Ziyang and Ningshan county of China highlight a higher prevalence of benign thyroid disease in individuals with low selenium status, although the optimal intake range remains narrow, cautioning against widespread selenium supplementation [17]. The calculated Chi-square test value of 8.59 (p=0.0034) in the present study indicates a statistically significant association between autoimmune thyroid disease and selenium status in the studied population. This finding supports the notion that selenium status may play a role in the development or progression of AITD, as observed in other case-control, cross-sectional and prospective studies as well.
Review of 17 articles, which included a total of 1,911 AITD subjects, noted a significant reduction in free triiodothyronine, free thyroxine and anti-thyroid peroxidase antibody after selenium supplementation. This indicates that selenium levels influence the thyroid functions, especially among patients with AITD [18]. Similarly, a meta-analysis of prospective studies where Hashimoto’s thyroiditis patients are followed-up with levothyroxine treatment, showed that selenium supplementation for three months significantly reduced TPOAb levels, along with improvements in mood and general well-being [19]. Low serum selenium has stated as risk factor for Hashimoto’s thyroiditis [17].
Limitation(s)
While the OR suggests a positive relationship between AITD and low selenium values observed in the present study, it does not establish a cause-and-effect relationship. Other confounding factors or variables not accounted for in the analysis could influence the observed association. As the sample size of the study is small, this has led to wider confidence intervals and less precise estimates. Additionally, the study population’s characteristics may limit the generalisability of the findings to other populations with different demographics or health profiles.
Conclusion(s)
The present study demonstrates that patients with AITD have significantly lower serum selenium levels compared to euthyroid controls, with selenium deficiency strongly associated with an increased risk of AITD (OR=8.60, RR=1.95). These findings suggest that selenium deficiency significantly contributes to the development and progression of AITD, emphasising the importance of selenium assessment in thyroid disorder management.