Carcinoid tumours are uncommon neuroendocrine growths known for their slow growth, often remaining asymptomatic until they metastasise or cause carcinoid syndrome. Recent studies suggest that their incidence is increasing, challenging their previous perception as benign and highlighting their potential for malignancy. These tumours originate from different parts of the gastrointestinal tract during embryonic development. Foregut carcinoids typically originate in the lungs, bronchi, or stomach; midgut carcinoids arise in the small intestine, appendix, or upper large bowel; and hindgut carcinoids develop in the lower colon or rectum. Carcinoid syndrome, a rare complication, is most commonly associated with midgut carcinoid tumours. The diagnosis of carcinoid tumours frequently occurs unexpectedly during unrelated surgical procedures. The choice of treatment and the prognosis are influenced by where the tumour is located and the extent of metastasis identified at the time of diagnosis. The present case is a case of a 65-year-old female with a major complaint of abdominal pain that had been progressive in nature for 2.5 years. She had a positive history of leprosy and tuberculosis. The diagnosis was confirmed by Contrast Enhanced Computed Tomography (CECT) and she was managed by exploratory laparotomy with ileocolic anastomosis.
Abdominal pain, Carcinoid syndrome, Colon tumours, Gastrointestinal tumours, Ileocolic anastomosis
Case Report
A 65-year-old female presented with complaints of abdominal pain for the last 2.5 years at the Department of Surgery, Jawaharlal Nehru Medical College, DMIHER, Sawangi, Meghe, Wardha, Maharashtra, India. The patient was apparently well before this period when she began experiencing pain in the abdomen, which was acute in onset, intermittent and pinpoint in nature. The pain gradually progressed, having no aggravating or relieving factors and was accompanied by reduced bladder emptying, nausea and vomiting. Her medical history was negative for seizures, fever, bowel complaints, cough, breathlessness, chest pain, rectal bleeding and weight loss. A history of tuberculosis and leprosy (50 years ago), for which she completed a course of medication, was noted. There were no previous complaints or symptoms of obstruction.
Per-rectal examination showed no external skin tags or any external masses, with normal anal tone and mucosa. There were no signs of fissures, internal fistulas, ballooning, or returning fingers stained with stool. The patient was further screened by proctoscopy, which revealed no signs of internal bleeding, no mass and no evidence of internal haemorrhoids.
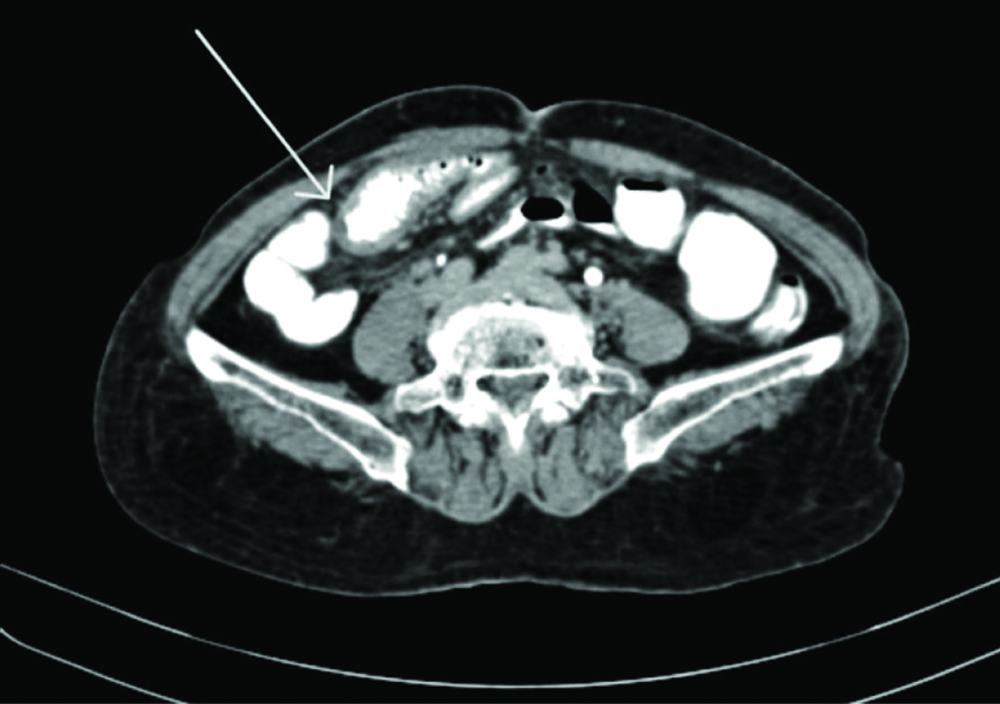
Ultrasonography (USG) was suggestive of a short segment of intussusception involving the ileum, which was observed in the right iliac fossa, alongside a suspicious 10×8 millimetre lesion adjacent to a lymph node. Mild thickening of the small bowel wall was noted in the same region. The remainder of the bowel appeared normal, with mild free fluid in the pelvis that could not be aspirated. The rest of the findings were consistent with previous scans. CECT of the abdominopelvic region suggested thickening of the wall of the distal ileum with minimal contrast within the lumen and several small mesenteric lymph nodes nearby, which suggested a possible stricture [Table/Fig-1]. The differential diagnosis included Ogilvie syndrome, ileoileal intussusception, irritable bowel syndrome, gastrointestinal motility disorders, coeliac disease, bowel adenocarcinoma and tumour lysis syndrome and a provisional diagnosis was designated as intussusception.
Contrast Enhanced Computed Tomography (CECT) showing mural thickening of distal ileal bowel loops with very thin of contrast within the lumen and multiple subcentric mesenteric lymph node in the adjacent area possibility of stricture.
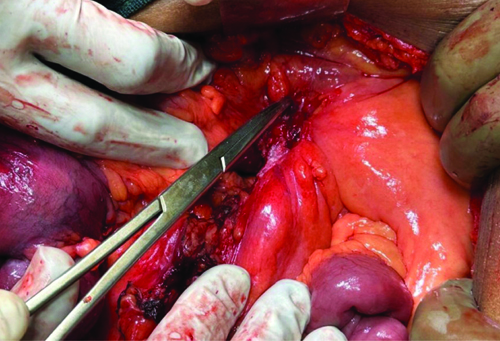
The patient was planned for exploratory laparotomy with ileocolic anastomosis. Intraoperative findings showed a normal omentum, but a thickened and fibrotic stricture mass was palpated at the ileocolic junction, encompassing the terminal ileum, cecum and a portion of the ascending colon. The mass was adherent to the retroperitoneum and positioned over the abdominal aorta. Tissue suspected to be adherent to the aorta was sent for histopathological analysis, which was indicative of a potential infective etiology. Based on the surgeons’ intraoperative expertise and clinical diagnosis, the ileoileal mass was resected with sufficient margins, followed by ileocolic anastomosis [Table/Fig-2,3].
Intraoperative picture showing ileoileal stricture.
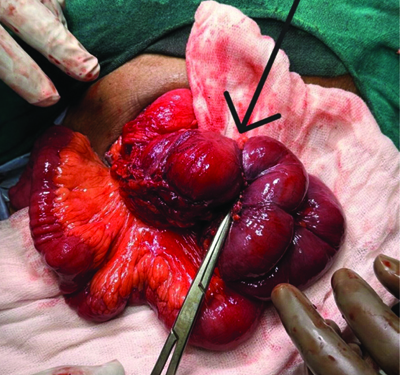
Intraoperative specimen delivered out showing ileoileal stricture.
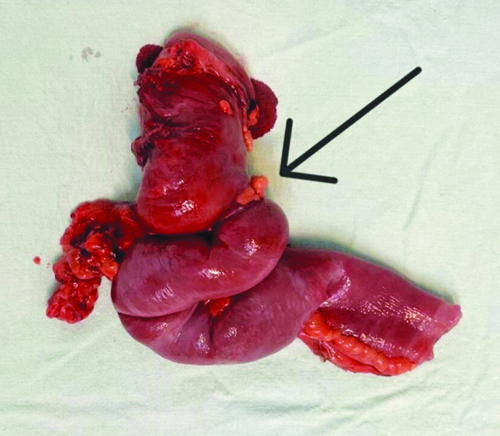
The resected specimen, measuring 25 centimetres in length, was sent for histopathological study, revealing features consistent with a well-differentiated Neuroendocrine Tumour (NET) of carcinoid type 1. The tumour was found to invade the visceral peritoneum (serosa) and exhibited a mitotic count ranging from 2 to 20 per cubic millimetre. Pathological Tumour, Node Metastasis (TNM) staging classified it as Tumour stage 4, lymph Node nil, Metastasis nil (pT4 NxMx), corresponding to AJCC stage IIIA, as per the clinical updates by Chauhan A et al., 2024 [Table/Fig-4,5] [1].
Resected specimen of small intestine cecum and ascending colon with adequate margins.
Section from the tumour shows carcinoid morphology; organoid growth (black box), uniform polygonal cells, finely granular ‘salt and pepper’ chromatin, inconspicuous nucleoli and abundant eosinophilic cytoplasm (yellow arrow) {Haematoxylin and Eosin (H&E), 10x}.

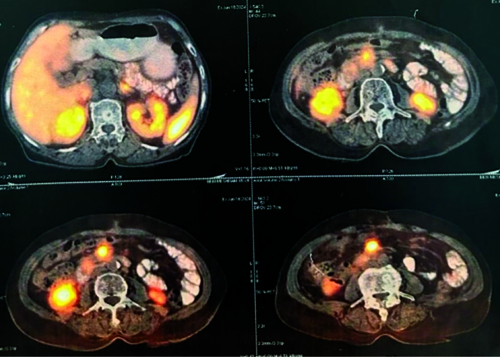
The patient was discharged and advised to follow-up with a Gallium-68 DOTATATE (DOTA) scan. The patient returned to the Surgical Outpatient Department (OPD) after two months with a DOTA scan report indicating a Somatostatin Receptor (SSTR) expressing lesion in a 4.4 centimetre segment of a small bowel loop, likely representing the primary mitotic lesion. Additionally, an SSTR receptor expressing lesion was observed in the right lobe of the liver, indicative of metastasis. No other SSTR expressing lesions were detected in the scanned areas of the body [Table/Fig-6,7]. Immunohistochemical markers were not carried out due to financial constraints. The patient was prescribed Imatinib 400 mg once a day for one month, along with a vitamin B12 supplement post-DOTA treatment.
DOTA-Positron Emission Tomography Computed Tomography (PET CT) scan report for assessment of tumour.
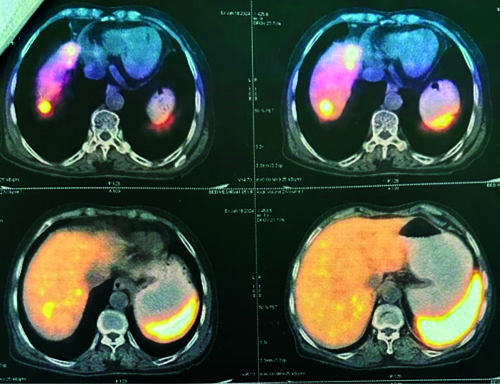
DOTA-PET CT scan report indicating a Somatostatin Receptor (SSTR) expressing lesion in a segment of a small bowel loop.
Discussion
The NET of the ileum is a specific type of tumour arising from neuroendocrine cells in the gastrointestinal tract. Carcinoid tumours were initially documented by Lubarsch more than a century ago, discovered during the autopsy of two patients [2]. They make up a minority, approximately 2 to 5%, of all cancers affecting the gastrointestinal tract. Siegfried Obendorfer, a German pathologist, introduced the term “karzinoide,” meaning ‘carcinoma-like,’ to describe tumours that exhibit a microscopic resemblance to carcinoma but behave benignly [3]. Rapport M et al., isolated and named serotonin {5-hydroxytryptamine (5-HT)}, which was considered a vasoconstrictive substance present in the blood. This was supported by other researchers who mentioned that Kulchitsky cells were the source of this amine. Later, it was discovered that ileal carcinoids are primarily responsible for carcinoid syndrome [4,5]. Histologically, carcinoid tumours are diagnosed through positive staining for chromogranin A and synaptophysin using Immunohistochemistry (IHC). These markers indicate neuroendocrine differentiation, a defining characteristic of carcinoid tumours. Moreover, the Ki-67 labelling index and mitotic index are critical for assessing the growth rate and proliferative activity of the tumour cells. These measures are used to classify NETs into different grades based on their potential aggressiveness and rate of growth [5-7]. Differential diagnoses to consider include Ogilvie syndrome, irritable bowel syndrome, ileoileal intussusception, gastrointestinal motility disorders, coeliac disease, bowel adenocarcinoma and tumour lysis syndrome due to similar clinical presentations.
Nodal and mesenteric metastases result from small bowel carcinoids triggering local fibrosis and ischaemia, which can potentially lead to small bowel obstruction. The surgical approach for patients with small bowel carcinoids depends on factors such as tumour size, location and metastatic spread [7,8]. Segmental resection followed by close monitoring is usually sufficient for tumours that are less than 2 centimetres without regional lymph node involvement. In contrast, tumours greater than 2 centimetres, those with metastatic lymph nodes in their vicinity and those with mesenteric spread may require a more aggressive surgical management approach. This involves surgical resection of the small bowel along with the related mesentery and lymph nodes, which has been linked to extended periods of disease-free survival [8,9]. Despite the primary small bowel tumour often being smaller than regional lymphadenopathy or distant metastases in aggressive cases, removing the primary tumour remains beneficial, potentially improving outcomes even when distant disease is present. Cytoreductive surgery plays a crucial role in managing widespread metastatic disease by aiming to alleviate symptoms and enhance survival through the removal or destruction of disseminated tumour metastases [7,8].
While aggressive surgical debulking may not offer a cure, research studies indicate that it has the potential for palliative benefits. A meta-analysis of patients with metastatic carcinoid disease in advanced stages reported complete resolution of carcinoid symptoms in over 80% of cases and a 5-year survival rate of over 70% when treated with cytoreductive partial hepatectomy. This finding is also supported by research conducted by Boudreaux JP et al., which noted that cytoreductive therapy provided relief from mesenteric ischaemia in over 80% of cases, which resulted from the encasement of the mesentery by the carcinoid tumour [10-12]. In summary, carcinoid tumours are rare cancers in the gastrointestinal tract characterised by their neuroendocrine features. Their diagnosis and classification rely on specific histological markers and measures of cellular proliferation.
Conclusion(s)
Carcinoid tumours are uncommon, slow-growing tumours with metastatic potential. They are usually diagnosed in later stages due to asymptomatic presentations or non specific symptoms. The prognosis of ileal carcinoid tumours can be favourable with early detection and timely treatment. Furthermore, multimodal treatment strategies, which include various therapeutic approaches such as surgery, chemotherapy and targeted therapies, have shown promising outcomes in patients with carcinoid tumours even at later stages of the disease, as evidenced by improved survival rates and quality of life. By systematically exploring optimal timing and effective combinations of treatment strategies, researchers can better tailor these strategies to individual patients, potentially extending survival and improving outcomes in this challenging tumour type.
[1]. Chauhan A, Chan K, Halfdanarson TR, Bellizzi AM, Rindi G, O’Toole D, Critical updates in neuroendocrine tumors: Version 9 American Joint Committee on Cancer staging system for gastroenteropancreatic neuroendocrine tumorsCA Cancer J Clin 2024 74(4):359-67.10.3322/caac.2184038685134 [Google Scholar] [CrossRef] [PubMed]
[2]. Howe JR, Carcinoid tumors: Past, present, and futureIndian J Surg Oncol 2020 11(2):182-87.10.1007/s13193-020-01079-632523259PMC7260338 [Google Scholar] [CrossRef] [PubMed]
[3]. Tsoucalas G, Karamanou M, Androutsos G, The eminent German pathologist Siegfried Oberndorfer (1876-1944) and his landmark work on carcinoid tumorsAnn Gastroenterol 2011 24(2):98-100. [Google Scholar]
[4]. Rapport M, Green A, Page I, Serum vasoconstrictor, serotonin; isolation and characterizationJ Biol Chem 1948 176(3):1243-51.10.1016/S0021-9258(18)57137-418100415 [Google Scholar] [CrossRef] [PubMed]
[5]. Pinchot SN, Holen K, Sippel RS, Chen H, Carcinoid tumorsOncologist 2008 13(12):1255-69.10.1634/theoncologist.2008-020719091780PMC2901509 [Google Scholar] [CrossRef] [PubMed]
[6]. Öberg K, Diagnosis and treatment of carcinoid tumorsExpert Rev Anticancer Ther 2003 3(6):863-77.10.1586/14737140.3.6.86314686708 [Google Scholar] [CrossRef] [PubMed]
[7]. Maroun J, Kocha W, Kvols L, Bjarnason G, Chen E, Germond C, Guidelines for the diagnosis and management of carcinoid tumours. Part 1: The gastrointestinal tract. A statement from a Canadian national carcinoid expert groupCurr Oncol 2006 13(2):67-76.10.3390/curroncol1302000617576444PMC1891174 [Google Scholar] [CrossRef] [PubMed]
[8]. Townsend CM Jr, Evers BM, Current management of gastrointestinal carcinoid tumorsJ Gastrointest Surg 2004 8(6):742-56.10.1016/j.gassur.2004.04.01015358337 [Google Scholar] [CrossRef] [PubMed]
[9]. Hellman P, Lundström T, Öhrvall U, Eriksson B, Skogseid B, Öberg K, Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastasesW J Surg 2002 26:991-97.10.1007/s00268-002-6630-z12016480 [Google Scholar] [CrossRef] [PubMed]
[10]. Que FG, Sarmiento JM, Nagorney DM, Hepatic surgery for metastatic gastrointestinal neuroendocrine tumorsCancer Control 2002 9(1):67-79.10.1177/10732748020090011111907468 [Google Scholar] [CrossRef] [PubMed]
[11]. Landry CS, Scoggins CR, McMasters KM, Martin RC, Management of hepatic metastasis of gastrointestinal carcinoid tumorsJ Surg Oncol 2008 97(3):253-58.10.1002/jso.2095718264984 [Google Scholar] [CrossRef] [PubMed]
[12]. Boudreaux JP, Putty B, Frey DJ, Woltering E, Anthony L, Daly I, Surgical treatment of advanced-stage carcinoid tumors: Lessons learnedAnn Surg 2005 241(6):839-46.10.1097/01.sla.0000164073.08093.5d15912033PMC1357164 [Google Scholar] [CrossRef] [PubMed]