The DPLDs are a group of disorders that involve the space between alveolar epithelial and capillary endothelial basement membranes [1]. The most important part of the work-up for patients with DPLD is detailed and proper history-taking to identify possible aetiologies, including occupational or drug exposures and signs of conditions like Connective Tissue Diseases (CTDs), sarcoidosis and infection [2]. The HRCT of the chest is the gold standard modality for DPLD diagnosis. It provides ten times more resolution than conventional imaging, revealing details that cannot otherwise be visualised [3].
DPLDs are often diagnosed clinico-radiologically and the follow-up of these patients involves repeat imaging, which raises concerns about radiation exposure. Spirometry with DLCO may help to address this issue, as it is radiation-free, easy to perform and can be repeated as needed [10].
PFTs are an easy and helpful tool for screening pulmonary vasculopathy in scleroderma patients. They assist in recognising early pulmonary hypertension, which can subsequently be confirmed with further testing [11]. However, pulmonary hypertension remains an important prognostic factor in these patients and it is usually detected using two-dimensional echocardiography with Doppler study. The FVC/DLCO ratio is a novel parameter, albeit one that is still little explored and is primarily used in systemic sclerosis.
This gap in the literature is addressed in this study, which aimed to evaluate the accuracy of the FVC/DLCO ratio in predicting the presence of pulmonary hypertension in patients with DPLD.
Materials and Methods
The cross-sectional time-bound study was conducted at NRS Medical College, Kolkata, West Bengal, India, in the respiratory medicine department (IPD and OPD) from January 2023 to March 2024. This study was approved by the IEC (NRSMC/IEC/178/2022). Written informed consent was obtained from every participant. A total of 50 patients diagnosed with DPLD during the study duration were included in the study.
Inclusion criteria: Patients older than 12 years of age with DPLD, either newly or previously diagnosed, who presented with clinical features suggestive of DPLD, such as dry cough, progressive exertional dyspnoea and other symptoms related to the aetiology, as well as radiological evidence on HRCT thorax of different patterns pertaining to DPLDs, were included.
Exclusion criteria: Patients with hepatic, renal, or cardiac co-morbidities that may cause pulmonary venous congestion or affect the pulmonary interstitium, thereby interfering with adequate effort and confounding the results during spirometry with DLCO. Patients recovering from major thoracic, abdominal, head, or ocular surgery. Patients with co-existing neuromuscular diseases and active haemoptysis. Patients with bullous airway diseases evident on HRCT thorax, a likelihood of pneumothorax based on clinical examination and those with significant kyphoscoliosis that may not be attributable to the disease process based on history. Additionally, patients on antitubercular drugs and those experiencing acute exacerbation of DPLD were also excluded from the study.
Study Procedure
Data were collected using case record forms after obtaining informed consent. All patients underwent HRCT thorax and spirometry with DLCO. A general physical examination and history-taking were conducted for all patients, particularly focusing on their smoking history. Some patients underwent serological testing, including Anti-Nuclear Antibody (ANA), ANA profile, Rheumatoid Factor (RF) and anti-Cyclic Citrullinated Peptide (CCP) for CTD-DPLD. Serum Angiotensin Converting Enzyme (ACE), 24-hour urinary calcium and serum calcium were tested in suspected cases of sarcoidosis. For patients with hypersensitivity pneumonitis, a serum hypersensitivity pneumonitis panel was conducted. Bronchoscopy with Broncho-Alveolar Lavage (BAL) fluid analysis was performed for cytology and to rule out infections, including tuberculosis, in some patients. All patients underwent ECHO 2D with Pulmonary Artery Systolic Pressure (PASP) estimation in the department of cardiology. Purposive, non random sampling was performed and patients were selected after being vetted through the inclusion and exclusion criteria.
The parameters studied included a description of the imaging findings on HRCT thorax images and radiological patterns such as UIP, NSIP, organising pneumonia, lymphocytic interstitial pneumonia, etc. Additionally, parameters such as FEV1, FVC, FEV1/FVC ratio and DLCO were noted. ECHO 2D with Doppler studies, with an emphasis on PASP, was also recorded. The 6MWD values were noted in all patients.
Statistical Analysis
Data were entered into MS Excel and analysed using MS Excel and GraphPad Prism software, version 9. Descriptive statistics, such as age distribution, smoking status, gender representation and the distribution of FEV1, FVC, DLCO and 6MWD values, were calculated using the descriptive statistics functions of MS Excel. The tests used included an unpaired t-test and simple logistic regression. A p-value <0.05 was considered statistically significant for this study. Simple logistic regression was performed between the FVC/DLCO ratio and the presence or absence of pulmonary hypertension, based on ECHO 2D with Doppler, as the binary outcome. The ROC curve was obtained and the FVC/DLCO cut-off ratio was adjusted to achieve the highest sensitivity for predicting pulmonary hypertension based on this dataset.
Results
In this study, of the 50 patients, 20 were male (40%) and the remaining 30 were female (60%). The mean age was 49.22±15.64 years. The most common age group was between 45 and 60 years, with 19 patients (38%) [Table/Fig-1]. Out of the total 50 patients, 15 (30%) were ex-smokers or current smokers, while the remaining 35 (70%) were non smokers.
| Age (in years) | Total number (n=50) |
|---|
| Mean±SD | 49.22±15.64 |
| Range | 15-79 |
| Age groups (in years) | n (%) |
| 15-30 | 7 (14) |
| 30-45 | 11 (22) |
| 45-60 | 19 (38) |
| 60-75 | 11 (22) |
| >75 | 2 (4) |
The most common HRCT thorax involvement was honeycombing (21 patients, 42%), followed by predominant reticulation and Ground Glass Opacity (GGO) with subpleural sparing (17 patients, 34%) [Table/Fig-2].
CT thorax description of major pattern (n=50).
| CT thorax description of major pattern | Number (%) |
|---|
| Honeycombing | 21 (42) |
| GGO, subpleural sparing with reticulation | 17 (34) |
| Other patterns | 12 (24) |
Other patterns included: two cases with bilateral peripheral predominant subpleural consolidation in a broncho-vascular distribution; four cases with peribronchovascular and fissural nodules with upper lobe predominant reticulation; two cases with bilateral multilobar GGO; one case with bizarre-shaped cysts in the lung with bilateral diffuse involvement; one case with lower lobe predominant cystic spaces with para-mediastinal cysts; one case with basal honeycombing and upper lobe predominant emphysema; and one case with cysts superimposed on GGO in a lower lobe predominant distribution with subpleural sparing. The most common overall pattern was the UIP pattern on HRCT thorax [Table/Fig-3].
CT thorax final radiological pattern (n=50).
| Radiological pattern | Number (%) |
|---|
| Usual Interstitial Pneumonia (UIP) | 19 (38) |
| Non Specific Interstitial Pneumonia (NSIP) | 11 (22) |
| Perilymphatic and fissural nodules | 5 (10) |
| Fibrosis with mosaic attenuation predominantly involving upper lobes | 3 (6) |
| Other patterns | 12 (24) |
Other patterns included one case of Combined Pulmonary Fibrosis with Emphysema (CPFE), three cases of cystic DPLD, two cases of diffuse alveolar hemorrhage characterised by bilateral diffuse GGO, two cases of probable UIP, two cases of organising pneumonia characterised by peripheral pleural-based multifocal consolidation and two cases with an unclassifiable pattern. CTD-DPLD was the most common overall diagnosis (22 patients, 44%), followed by IPF (11 patients, 22%) [Table/Fig-4].
Final DPLD diagnosis (clinico-radiological and serology tests as applicable) (n=50).
| DPLD type | Number (%) |
|---|
| Idiopathic Pulmonary Fibrosis (IPF) | 11 (22) |
| Connective Tissue Disease Related DPLD (CTD-DPLD) | 22 (44) |
| Sarcoidosis | 5 (10) |
| Chronic hypersensitivity pneumonitis | 3 (6) |
| Idiopathic pulmonary haemosiderosis | 2 (4) |
| Pulmonary langerhans cell histiocytosis | 2 (4) |
| Cryptogenic Organising Pneumonia (COP) | 1 (2) |
| Combined Pulmonary Fibrosis with Emphysema (CPFE) | 1 (2) |
| Silicosis | 1 (2) |
| Unclassifiable | 2 (4) |
The mean DLCO adjusted for IPF patients was 8.5909±4.5302, while in non IPF patients, the mean DLCO adjusted was 13.8513±10.7887. The difference in means, evaluated using an unpaired t-test with Welch’s correction, was statistically significant (p-value=0.0217). The difference in means between 6MWD was also statistically significant (p-value=0.0028) [Table/Fig-5].
IPF vs non IPF 6MWD and DLCO (n=50).
| Type of DPLD | DLCO (in mL/min/mm Hg) mean±SD | 6MWD (m) mean±SD |
|---|
| IPF | 8.59±4.53 | 222.55±73.79 |
| Non IPF | 13.85±10.78 | 320.1±123.10 |
| p-value | 0.0217 | 0.0028 |
The most common spirometry curve pattern was restrictive (38 patients, 76%), followed by a mixed defect in eight patients (16%). The obstructive pattern was noted in two patients with sarcoidosis and one patient with pulmonary Langerhans cell histiocytosis, while one patient with idiopathic pulmonary haemosiderosis had a normal flow-volume loop [Table/Fig-6].
| Flow volume loop pattern | Number (%) |
|---|
| Restrictive | 38 (76) |
| Mixed | 8 (16) |
| Obstructive | 3 (6) |
| Normal | 1 (2) |
The difference in means was statistically significant for 6MWD and DLCO parameter, while it was not statistically significant for the FVC/DLCO ratio [Table/Fig-7]. No statistically significant difference in mean values was observed between the IPF and non IPF groups in FEV1, FVC values and the FEV1/FVC ratio [Table/Fig-8].
Pulmonary hypertension and 6MWD in metres (n=50).
| Presence of pulmonary hypertension on echo 2D | 6MWD (mean±SD)
in metres | FVC/DLCO ratio
(mean±SD) | FVC (mean±SD)
in mL | DLCO (mean±SD) in mL/min/mm Hg |
|---|
| Yes (37 out of 50 patients, 74%) | 298.86±128.23 | 1.98±1.49 | 1478.83±498.01 | 10.31±8.20 |
| No (13 out of 50 patients, 26%) | 298±100 | 1.46±1.02 | 1737.69±517.33 | 19.47±11.68 |
| p-value | 0.0004 | 0.1836 | 0.1216 | 0.0187 |
Unpaired t-test with Welch’s correction was used
Diagnosis and spirometry parameters (n=50).
| Diagnosis | FEV1 (Mean±SD) in mL | FVC (Mean±SD) in mL | FEV1/FVC ratio |
|---|
| IPF | 1216.34±439.14 | 1550.72±574.26 | 0.79±0.05 |
| Non IPF | 1230.35±355.16 | 1537.25±500.68 | 0.80±0.07 |
| p-value | 0.9240 | 0.9447 | 0.4027 |
Unpaired t-test was used
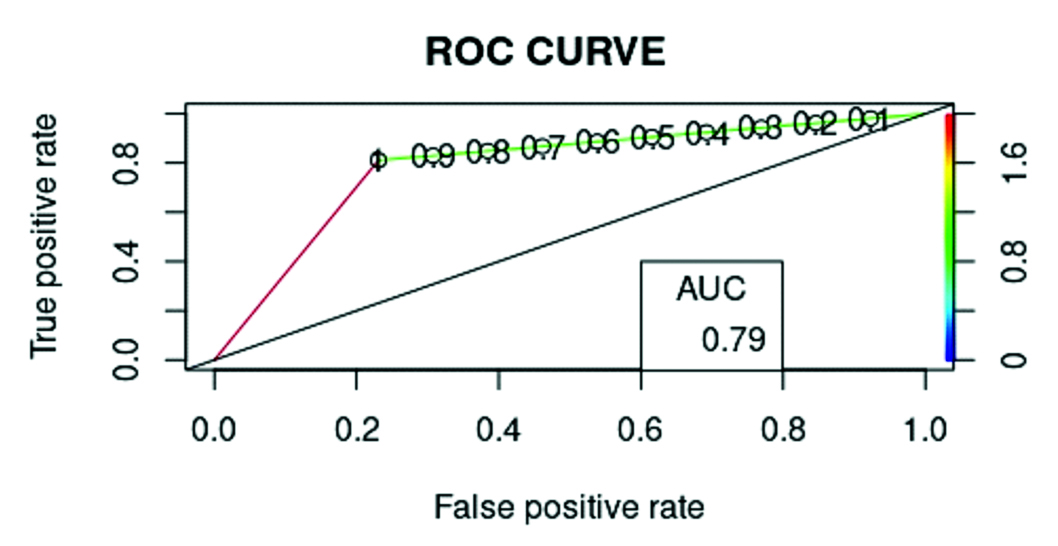
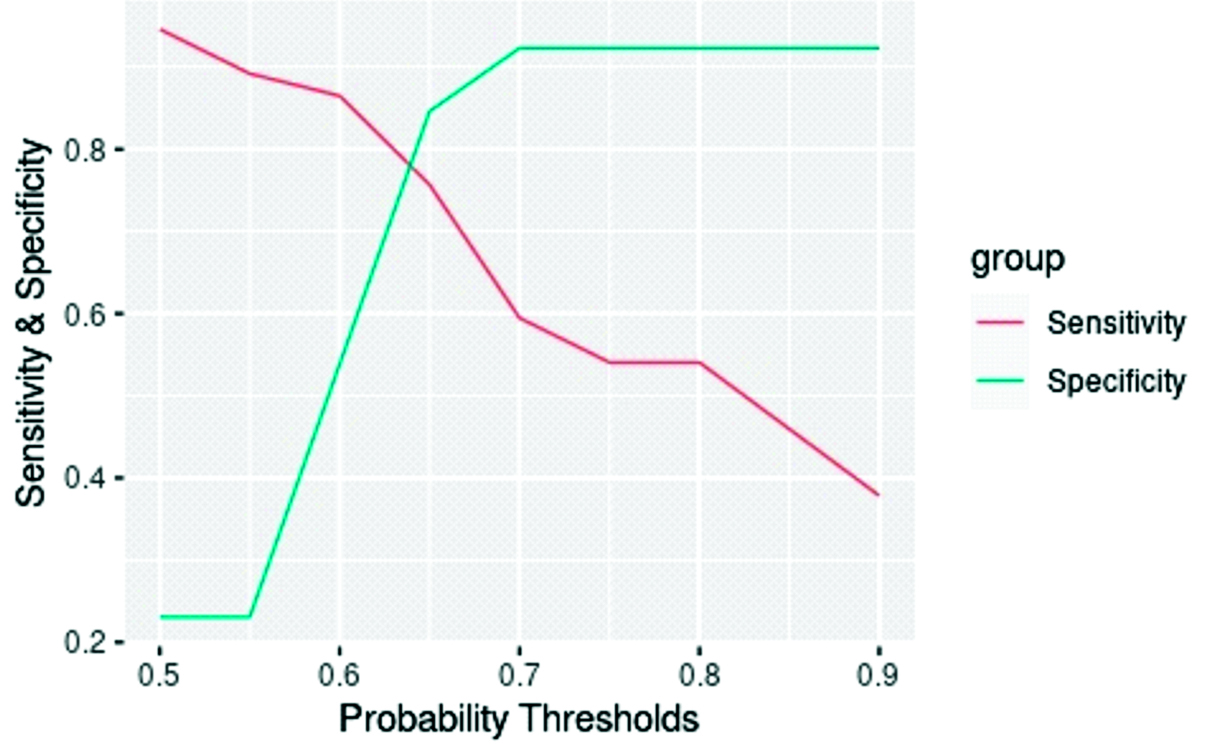
The FVC/DLCO ratio of patients with and without pulmonary hypertension was analysed using a simple logistic regression test. Since this ratio serves as a screening test, a probability threshold providing the highest sensitivity was chosen. A ratio of 0.97 was determined to predict the presence of pulmonary hypertension, while a value lower than that was used to infer the absence of pulmonary hypertension. Based on this dataset and model, the analysis reached a sensitivity of 81%, specificity of 77%, PPV of 86%, NPV of 70% and diagnostic accuracy of 80% [Table/Fig-9,10].
Sensitivity and specificity plot (n=50).
Discussion
The DPLDs are a heterogeneous group of diseases whose incidence increases with age [12]. This study was performed to gain insight into the functional status of DPLD patients using spirometry with DLCO, as well as an evaluation of pulmonary hypertension from the FVC/DLCO ratio, if feasible, thus attempting to supplement 2D- ECHO, which is often operator-dependent. The 6MWD was also conducted as part of the physiological assessment and is a valuable tool in assessing prognosis and evaluating the need for pulmonary rehabilitation [13], an often overlooked area in these patients.
In this study, female patients comprised 60% of the sample size, which contrasts with the findings published by Tentu AK et al., in which the majority of patients (72%) were male. A possible explanation for this finding could be a hospital-based selection bias [14]. The most common age group in this study was 45-60 years, with 19 patients (38%), which corroborates with Tentu AK et al.’s finding, where the most common age was around 50 years (78%) [14]. The most common spirometry loop pattern was restrictive (76%), which was similar to the findings of Balas Z et al., [15].
The most common DPLD in this study was CTD-DPLD, which comprised 22 out of 50 cases, or 44%. This contrasts with the findings of the Indian Interstitial Lung Disease (ILD) registry, which observed that the most common ILD was hypersensitivity pneumonitis, followed by CTD-ILD and Idiopathic Pulmonary Fibrosis (IPF) [16]. This contrasting finding in present study could be explained by hospital-based bias and the limited sample size used in this study, in contrast to the Indian DPLD registry of 1,084 patients. The prevalence of CTDs was found to be higher in association with DPLD than in the general population [17]. The most common DPLD CT involvement was honeycombing with subpleural involvement and GGO with reticulation (38 cases, 76%). Xaubet A et al., described a moderate correlation between abnormalities on HRCT thorax in 39 treated IPF patients and their corresponding DLCO and FVC values [18]. There was no statistically significant difference in the mean FEV1, FVC and FEV1/FVC ratio between the IPF and non IPF groups, which could be due to the heterogeneous nature of patients in the groups, sampling bias, non randomisation and the discrepancy in subgroup sample size.
Out of a total of 50 patients, 15 (30%) had exposure to smoking, while the remaining 35 (70%) were never smokers, which contrasts with the findings of Patel S et al., where a smoking proportion of 61% among the study population was reported [19]. The difference in 6MWD between patients with pulmonary hypertension and those without corroborated the findings of Andersen CU et al., in which, out of 212 patients, a 6MWD of less than 345 m was independently associated with pulmonary hypertension [20]. The FVC/DLCO ratio is a novel parameter and is mostly described for systemic sclerosis DPLD. In this study, an FVC/DLCO ratio of 0.97 predicted pulmonary hypertension as evaluated on ECHO2D, with a sensitivity of 81%, specificity of 77%, a positive predictive value of 86%, a negative predictive value of 70% and a diagnostic accuracy of 80%. This was in contrast to the cut-off of 1.39 as advocated by Eid D et al., [21]. However, the latter study only dealt with patients with systemic sclerosis and pulmonary hypertension, while this study included different DPLD patients with and without pulmonary hypertension.
Limitation(s)
The study was conducted at a single centre in a tertiary care hospital, so hospital bias cannot be ruled out. It was carried out in the IPD and OPD of respiratory medicine, which may introduce an element of selection bias. Additionally, since this was a cross-sectional study, it was not possible to assess prognosis.
Conclusion(s)
The most common DPLD encountered in the study was connective tissue disease-associated DPLD (CTD-DPLD), followed by IPF and sarcoidosis, in that order. An FVC/DLCO ratio of 0.97 serves as a good cut-off for predicting pulmonary hypertension in these patients.
Unpaired t-test with Welch’s correction was used
Unpaired t-test was used