The most commonly employed mode of anaesthesia for surgeries involving the lower limb, perineum, and lower abdomen is spinal anaesthesia [1]. Plain bupivacaine blocks voltage-gated sodium channels on the neuronal membrane and provides a blockade, but it has disadvantages such as a shorter duration and less postoperative analgesia. To address this, a number of adjuvants, including midazolam, fentanyl, tramadol, nalbuphine, and magnesium sulphate, have been introduced to improve postoperative analgesia, prolong the time required for the reversal of the block, and accelerate the onset of the block [1,2]. Although effective, neuraxial opioids have a number of unfavorable side effects [3,4].
Current research has focused on non opioid spinal receptors that alleviate the transmission of pain impulses. Lower limb and pelvic orthopaedic surgeries require adequate motor and sensory blockade for effective surgery, as well as sufficient postoperative analgesia for patient comfort. To prolong postoperative analgesia in a less cumbersome way, as a feasible alternative to tedious and costly techniques (such as epidural anaesthesia), author can add potent adjuvants like alpha-2 adrenergic receptor agonists in effective and safer doses intrathecally [5-7].
When alpha-2 adrenergic agonist medications are injected concurrently, regional vasoconstriction occurs, and blockade of C fibers is facilitated. This spinal action may be achieved through retrograde transport along the axon or simple diffusion alongside the nerve, which enhances the effectiveness of local anaesthetics in inhibiting pain signals [8]. As an additive to intrathecal local anaesthesia, a more selective alpha-2 adrenoceptor agonist, such as dexmedetomidine, has been used in recent times [8,9]. The goal was to determine the optimal dosage of dexmedetomidine between 5 μg and 10 μg for an average adult Indian population by comparing and identifying which dosage is both safe and effective. Similar studies have been conducted in the past with different dosages of bupivacaine, but author have assessed all parameters to ascertain which dosage of dexmedetomidine is superior as an adjuvant with 3 mL of hyperbaric bupivacaine in orthopaedic surgeries [10,11]. The primary goals of the study were to determine the onset and duration of sensory and motor block, as well as the duration of postoperative analgesia. The additional goals of the study were to assess haemodynamic parameters and any complications or side effects.
Materials and Methods
This randomised clinical and single-blind study was conducted at the OT complex of Dheeraj Hospital, SBKS, and MIRC, Sumandeep Vidyapeeth, Vododhara, Gujarat, India in the Department of Anaesthesia over a period of 18 months, from September 2022 to March 2024. Author included patients of both genders, aged 18 to 65 years, who were scheduled for lower limb and pelvic orthopaedic surgeries requiring spinal anaesthesia. The Institutional Ethical Committee approved the study, with approval number SVIEC/ON/MEDI/BNPG21/SEP/22/9. After obtaining written informed consent, the consultant Anaesthesiologist involved in the study administered the medication.
Inclusion criteria: Patients aged between 18 and 65 years, of both genders, belonging to American Society of Anaesthesiology (ASA) Grade I and II, who were undergoing lower limb and pelvic orthopaedic surgeries and willing to sign a written informed consent form, were included in the study.
Exclusion criteria: Patients suffering from shock, septicaemia, anaemia, or uncontrolled hypertension; patients on any anticoagulant therapy or those with coagulation problems; patients with infections at the suggested puncture site for spinal anaesthesia; patients with spinal deformities; and patients allergic to local anaesthetic drugs, as well as those who refused to sign the written informed consent form, were excluded from the study.
Sample size: The sample size was calculated based on a pilot study that assessed the duration of sensory and motor block in the two groups. Considering a 15% difference, the derived sample size was 82, resulting in 41 patients in each group.
Allocation of groups: Randomisation was performed using a computer-generated random number table from StatTrek. Even numbers were allocated to group D1, while odd numbers were allocated to group D2, as shown in [Table/Fig-1]. Similar drug dosages were used in previous studies, such as those conducted by Srikanth I et al., [10].
Consodilated Standards of Reporting Trails (CONSORT) flow diagram.
Group D1 received 0.5% hyperbaric bupivacaine 3 mL+5 μg dexmedetomidine (0.5 mL)+0.5 mL sterile normal saline, making a total of 3.1 mL.
Group D2 received 0.5% hyperbaric bupivacaine 3 mL+10 μg dexmedetomidine (0.1 mL), also totaling 3.1 mL.
Study Procedure
All patients underwent necessary preoperative investigations, and any other special investigations required were ordered.
Prior to surgery, all patients were instructed to follow a Nil Per Oral (NPO) regimen for six hours for solid food and two hours for water. The acceptance of spinal anaesthesia was obtained through written informed consent. An 18G green intravenous (i.v.) catheter was placed, and i.v. fluids (Ringer’s lactate) were started as soon as the patient entered the operating room. The subjects were connected to a multichannel monitor. Intravenous (i.v.) injections of 0.2 mg of glycopyrrolate and 4 mg of ondansetron were administered as premedication to all patients, and each patient was preloaded.
Painting and draping of the patient’s back were performed while they were in a sitting position. Tuffier’s line, which passes across the L3 and L4 intervertebral spaces, was used to identify the level for spinal puncture. A 23G spinal needle was used, and the puncture was made in the midline at the L3-L4 interspace. The drug was administered at a speed of 0.2 mL per second following the free flow of clear Cerebrospinal Fluid (CSF). After the administration, the patient was positioned supine.
Assessment of parameters: The assessment of all parameters was conducted by an Anaesthesiologist other than the one who performed the procedure, ensuring single blinding regarding the drug dosage. The assessment of haemodynamic parameters {heart rate, Systolic Blood Pressure (SBP), and Diastolic Blood Pressure (DBP)} was conducted at intervals of 0, 5, 10, 15, 30, 45, 60, 75, and 90 minutes.
Sensory blockade assessment was conducted using the pinprick test [12]. Motor blockade assessment was performed using the Modified Bromage scale [13]. Analgesia was evaluated using the Visual Analogue Scale (VAS) [14].
The following parameters were assessed:
Onset of sensory block: The time from the intrathecal injection to the loss of pinprick sensation at the L1 level.
Duration of sensory block: The time from the intrathecal injection to the sensory regression to the S1 dermatome.
T10 level time.
Highest sensory level observed and the time taken to reach it.
Two-segment regression time.
Onset of motor block: The time taken from intrathecal injection to achieve grade 3 motor block.
Duration of motor block: The time taken from intrathecal injection to reach grade 0 motor block.
Duration of analgesia: The time taken from intrathecal injection to the administration of the first rescue analgesia. Rescue analgesia was given in the form of inj. Diclofenac 75 mg i.v. when VAS was greater than 3.
Complications: Bradycardia {Pulse Rate (PR) <60/min), hypotension {Mean Blood Pressure (MBP) <60 mmHg}, nausea, and vomiting.
Statistical Analysis
Differences between the data of the groups in the demographic data, observed data, and baseline values were analysed using statistical tests such as the unpaired t-test and Chi-square test. Numerical data are presented as mean±standard deviation, while categorical data are presented as percentages and frequencies. A p-value of <0.05 in the tests is considered statistically significant, and a p-value of <0.001 is considered highly significant.
Results
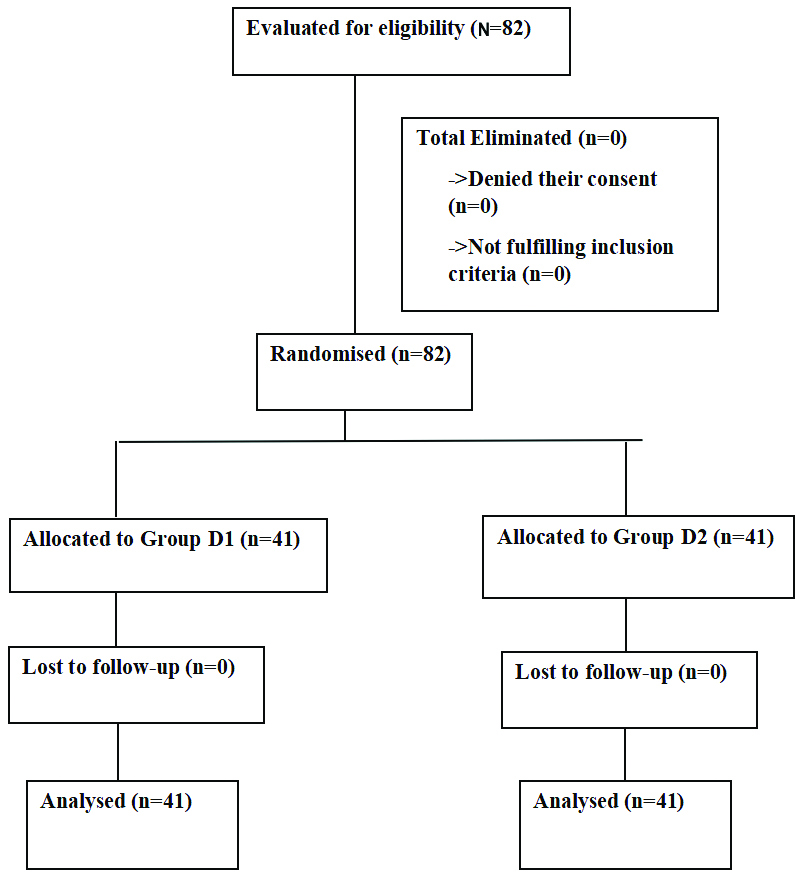
The demographic data were comparable in groups D1 and D2 and were statistically non significant (p-value >0.05), as shown in [Table/Fig-2]. The comparison of mean heart rate between the two groups showed a statistically significant difference at time intervals from 15 minutes to 60 minutes, with a p-value <0.05. Group D2 had a lower heart rate compared to group D1, as shown in [Table/Fig-3].
Demographic data (Unpaired t-test and Chi-square test).
| Parameters | Group D1 | Group D2 | p-value |
|---|
| Mean±SD | Mean±SD |
|---|
| Age (years) | 38.22±8.147 | 36.76±7.946 | 0.41 |
| Weight (kg) | 59.05±6.697 | 59.66±6.394 | 0.69 |
| Gender (Female/Male) n% | 12 (29.3%)/29 (70.7%) | 9 (22%)/32 (78%) | 0.44 |
| ASA (grade I/II) n% | 18 (43.9%)/23 (56.1%) | 21 (51.2%)/20 (48.8%) | 0.507 |
Comparison of heart rate between two groups (Unpaired t-test).
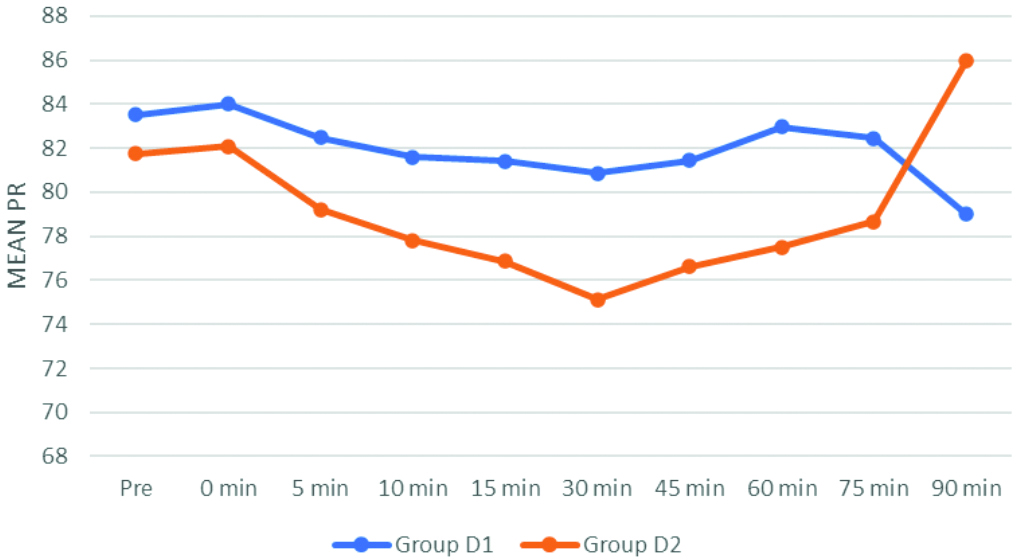
Comparison of mean SBP between the groups showed a statistically highly significant difference from 10 minutes to 60 minutes, with group D2 experiencing a significant fall in SBP compared to group D1, with a p-value <0.001 has been illustrated in [Table/Fig-4]. Additionally, the comparison of mean DBP revealed a statistically highly significant difference from five minutes to 60 minutes, where group D2 had a significant fall in DBP compared to group D1, also with a p-value <0.001.
Comparison of SBP and DBP between two groups (Unpaired t-test).
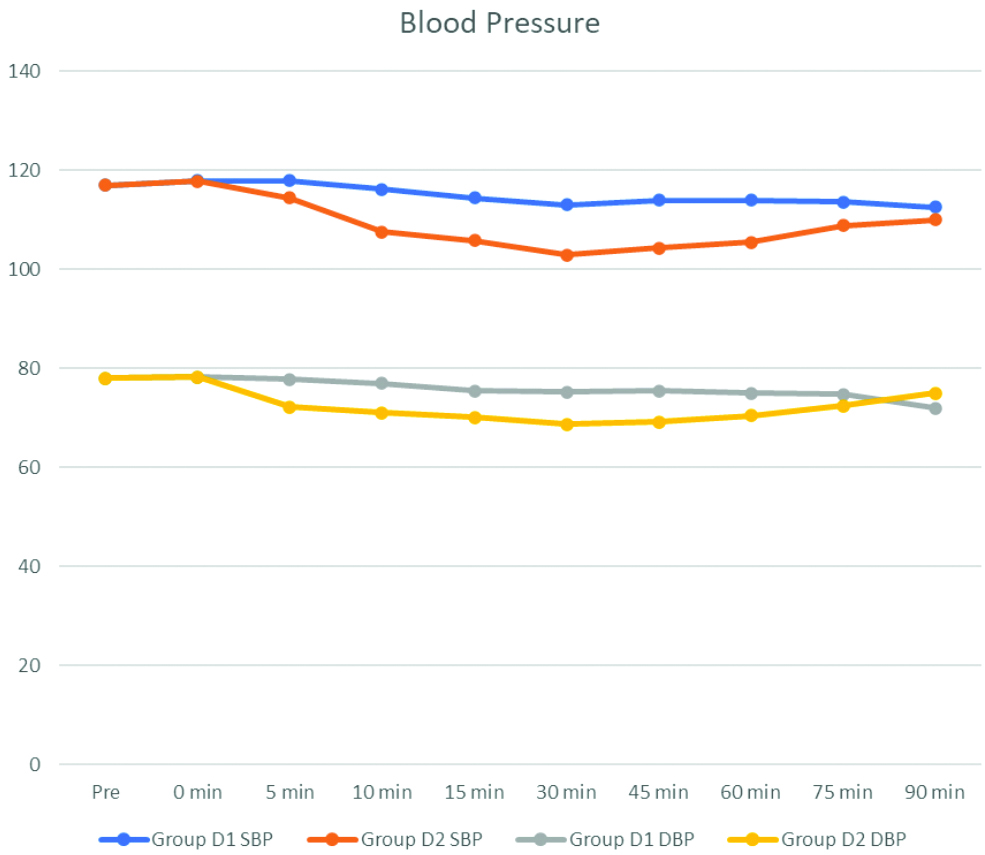
Statistically highly significant difference between the two groups for all the parameters measured has been depicted in [Table/Fig-5]. In group D1, 31 patients attained the T8 level, nine patients attained the T6 level, and only one patient attained the T4 level. In group D2, eight patients attained the T8 level, 28 patients attained the T6 level, and five patients attained the T4 level, as shown in [Table/Fig-6]. The difference was statistically highly significant (p<0.001).
Block characteristic (Unpaired t-test) (p<0.05* statistically significant, p<0.001** statistically highly significant).
| Characteristics of block | Group D1 | Group D2 | p-value |
|---|
| Mean±SD | Mean±SD |
|---|
| Onset of sensory block (min) | 2.87±0.36 | 2.01±0.12 | 0.001** |
| Time to achieve highest level (min) | 14.402±0.67 | 12.25±0.41 | 0.001** |
| 2 segment regression time (min) | 121.93±5.703 | 157.85±8.51 | 0.001** |
| Onset of motor block (min) | 3.36±0.43 | 2.63±0.26 | 0.001** |
| Duration of motor block (min) | 257.37±25.02 | 396.17±36.13 | 0.001** |
| Duration of sensory block (min) | 318.02±32.27 | 457.46±32.85 | 0.001** |
| Duration of analgesia (min) | 219.51±23.39 | 364.29±43.64 | 0.001** |
Highest sensory level achieved (Unpaired t-test).
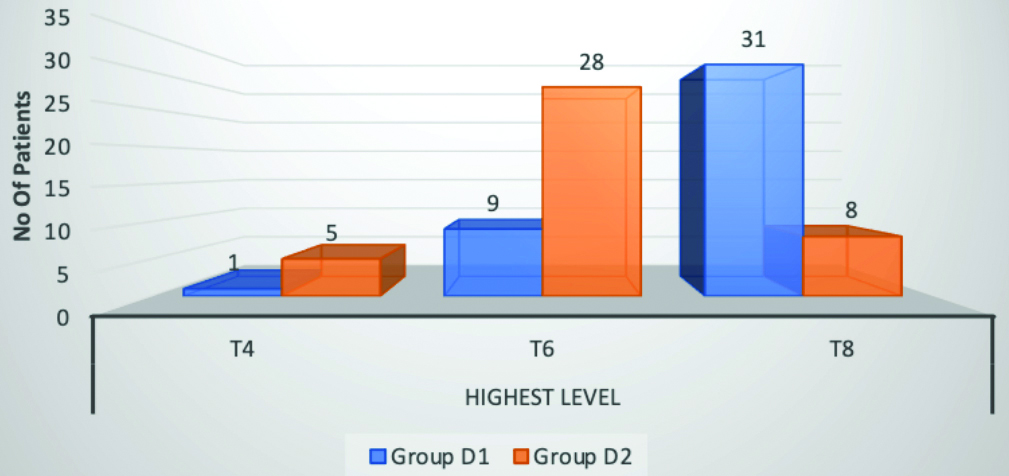
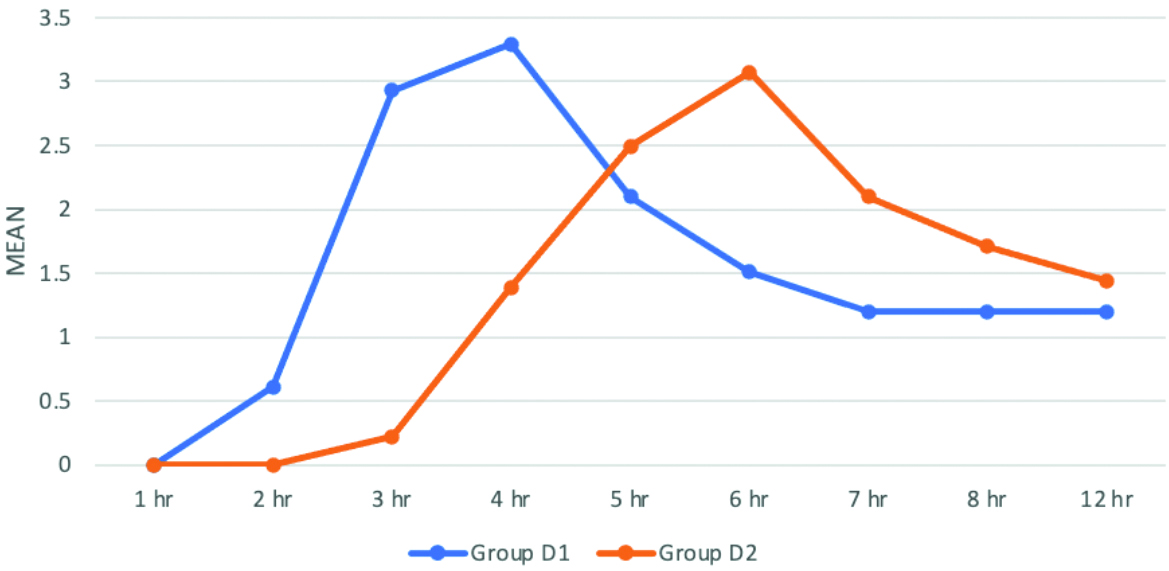
The VAS score comparison between the two groups showed statistically significant results (p-value <0.001). The time to achieve higher VAS scores was significantly delayed in group D2 compared to group D1. Intra group comparison was performed using repeated measures ANOVA, which was significant in group D1 (p value=0.04) and highly significant in group D2 (p-value <0.01), as shown in [Table/Fig-7]. None of the patients in either group developed any complications.
VAS comparison between two groups. (Unpaired t-test).
Discussion
Dexmedetomidine has emerged as the adjuvant medication with the greatest potential in regional anaesthesia due to its ability to enhance the pharmacodynamic properties of neuraxial procedures [15]. In present study, author compared the efficacy of two different doses of dexmedetomidine 5 μg and 10 μg- administered intrathecally as an adjuvant to 3 mL of hyperbaric bupivacaine used for spinal anaesthesia in lower limb and pelvic surgeries.
Socio-demographic data: In present study, confounding factors such as gender, ASA grading, age, and weight between the evaluating groups were not statistically significant, similar to the studies conducted by Bhure AR et al., and Gupta M et al., [16,17].
Haemodynamic parameters: In present study, there was a significant difference in the pulse rate during the intraoperative period. Baseline values were comparable. A significant decrease in pulse rate was noted in group D2 compared to group D1 during the intraoperative period; however, complications such as bradycardia (pulse rate less than 60 bpm) were not observed. Khan AL et al., conducted a comparative study between intrathecal 5 μg dexmedetomidine (dex) and 25 μg fentanyl as adjuvants to intrathecal bupivacaine in lower abdominal surgeries [18]. In comparison to the fentanyl group, Khan AL et al., deduced that the pulse rate in the dexmedetomidine group was decreased at all time intervals.
This research revealed a statistically significant difference between the intraoperative SBP and DBP values. A significant decrease in blood pressure values was noted with 10 μg dexmedetomidine during the intraoperative period. However, blood pressure values did not drop to a level that required the use of vasopressors. Srikanth I et al., found a modest reduction in the incidence of hypotension in the dexmedetomidine 5 μg group compared to the dexmedetomidine 10 μg group, although the results were insignificant [10]. Naaz S et al., conducted a study to determine the ideal intrathecal dose of dexmedetomidine for lower abdominal surgery [1]. They compared doses of dexmedetomidine—10 μg, 15 μg, and 20 μg—and found hypotension in the 15 μg and 20 μg groups but not in the 10 μg group, which is consistent with present findings.
Sensory block: The onset of sensory block was faster in Group D2 compared to group D1 in this study. Similar results were obtained in the study conducted by Gupta M et al., [17]. They divided 90 adult patients undergoing elective lower abdominal and lower limb surgeries into three groups to receive intrathecal 0.5% bupivacaine 3 mL with 2.5 μg (group BD 2.5), 5 μg (group BD 5), or 10 μg (group BD 10) dexmedetomidine in 0.5 mL normal saline. They concluded that the onset of sensory block was significantly earlier in group BD 10 compared with group BD 5 (p=0.035) and group BD 2.5 (p=0.010). The results of this study are also comparable to those obtained in the study conducted by Kapinegowda ST et al., [19]. They randomly allocated 90 adult patients undergoing infraumbilical surgeries into three groups to receive intrathecal 0.5% bupivacaine 2.5 mL with 5 μg (group BD 5), 10 μg (group BD 10), or 15 μg (group BD 15) dexmedetomidine in 0.5 mL normal saline. They found that the onset time of sensory block was 2.76±1.32 in group D5, 2.45±1.50 in group D10, and 1.86±0.93 in group D15, which is statistically significant (p=0.025). Thus, it can be concluded that increasing the dosage of dexmedetomidine shortens the onset time of sensory block.
In this study, the time to achieve a sensory level of T10 and the time to achieve the highest sensory level were shorter in group D2 compared to group D1, which were comparable to the results obtained by Naaz S et al., [1]. They designed groups with 2.5 mL hyperbaric bupivacaine with 0.5 mL saline (Control), or 0.5 mL dexmedetomidine: 5 μg (D1), 10 μg (D2), 15 μg (D3), and 20 μg (D4). Looking at the results of the D1 and D2 groups, the time to achieve a level of T10 was 6.55±0.97 in group D1 and 6.22±0.42 in group D2, while the time to achieve the highest sensory level was 14.43±3.11 in group D1 and 12.01±2.97 in group D2. In contrast, the study conducted by Srikanth I et al., showed that there was no statistically significant difference in the time to achieve the sensory level of T10 and the time to achieve the highest sensory level between the dexmedetomidine 5 μg and dexmedetomidine 10 μg groups, with a p-value>0.05 [10]. The dissimilarity in the results may be due to differences in study design.
In this study, the highest level achieved was T4 in both groups, as shown in [Table/Fig-6]. In group D1, the maximum number of patients achieved a T8 level, which included 31 patients, while in group D2, the maximum number of patients achieved a T6 level, which included 26 patients. T4 level was achieved by only one patient in group D1, whereas five patients in group D2 achieved T4 level. Likewise, a study conducted by Naaz S et al., showed that in the 5 μg group, the maximum number of patients achieved T8, with a range from T6 to T8, and in the 10 μg group, the maximum number of patients achieved T6, with a range from T4 to T8 [1].
In this study, the time of two-segment regression of sensory analgesia was significantly prolonged in group D2 compared to group D1. A similar result was obtained by Gupta M et al., [17]. They found that the time of two-segment regression of sensory analgesia was 149.17±21.66 in group BD5 and 187.50±24.56 in group BD10, with a p-value of <0.001. Bhure AR et al., conducted a comparative study of two doses of intrathecal dexmedetomidine (10 μg and 15 μg) as adjuvants to 0.5% hyperbaric bupivacaine for abdominal hysterectomy [16]. They concluded that dexmedetomidine prolongs the two-segment regression time of sensory analgesia in a dose-dependent manner (122±18.78 min in the 10 μg group and 137±16.48 min in the 15 μg group), which is consistent with present study.
The total duration of sensory block was significantly longer in group D2 compared to group D1. Al-Mustafa MM et al., conducted a study on the effect of dexmedetomidine added to spinal bupivacaine for urological procedures [20]. They randomly divided 60 patients into three groups, each receiving 12.5 mg of bupivacaine intrathecally combined with normal saline (group N), dexmedetomidine 5 μg (group D5), or dexmedetomidine 10 μg (group D10). The duration of analgesia was 338.9±44.8 minutes in group D10, 277.1±23.2 minutes in group D5, and 165.5±32.9 minutes in group N, with a p-value of <0.001 between the D5 and D10 groups, which is in accordance with this study. A similar result was also obtained by Kapinegowda ST et al., [19]. In their study, the duration of sensory analgesia was 319.83±61.41 in group D5, 336.13±61.38 in group D10, and 415.20±96.6 in group D15, with a p-value <0.001. Intrathecal dexmedetomidine exhibits prolongation of sensory block by a dual mechanism: inhibiting the release of neurotransmitters by acting on the presynaptic α2A receptors and hyperpolarising the post-synaptic neurons [21].
Motor block: In the current study, the time of onset of motor block was significantly faster in group D2 compared to group D1. A similar result was obtained by Koolwal P et al., [22]. In their study, 90 patients were divided into three groups receiving a total volume of 3.5 mL, which contained 3 mL of hyperbaric bupivacaine with 0.5 ml of 0.9% normal saline (group A), 5 μg dexmedetomidine (group B), and 10 μg dexmedetomidine (group C). They observed that the mean time of motor onset was significantly longer in group A compared to groups B and C, and groups-B and C also differed significantly (mean of groups-A > B > C). Reddy BS and Surya K, conducted a similar study to compare 5 μg and 10 μg dexmedetomidine as an adjuvant to bupivacaine. In their study, the onset of motor block was 3.86±0.50 minutes in the 5 μg group and 6.13±1.31 minutes in the 10 μg group, with a p-value of 0.001 [11]. They concluded that 10 μg dexmedetomidine significantly hastened the onset of motor block, which is consistent with the current study.
The duration of motor block was longer in group D2 than in group D1 in the current study. Similarly, the duration of motor block was also prolonged in the study conducted by Mowar A et al., [23]. They studied the effect of three different doses of intrathecal dexmedetomidine on subarachnoid block. They divided 90 patients into three groups: group A received 0.5% hyperbaric bupivacaine 12.5 mg (2.5 mL) with 2.5 μg dexmedetomidine, group B received 5 μg dexmedetomidine with bupivacaine, and group C received 10 μg dexmedetomidine with bupivacaine. The duration of motor block was 312±29.64 in group B and 361.4±16.14 in group C, with a p-value of <0.001. Singh AP et al., conducted a study with three different doses of dexmedetomidine: 5 μg, 7.5 μg, and 10 μg [24]. They found that the duration of motor block was significantly prolonged in the 10 μg group, with a p-value of <0.001, which is also in accordance with the results of the current study. The prolongation of motor block by intrathecal dexmedetomidine might be due to its inhibitory effect on the motor neurons in the dorsal horn of the spinal cord [25].
Analgesia and side effects: In the study, the time to achieve a higher VAS score was delayed in group D2 compared to group D1, and the need for rescue analgesia was also significantly delayed in group D2 compared to group D1. Similar results were obtained by Shaikh SI and Dattatri R, [26] in their study. They found that the time to the first analgesic rescue was significantly prolonged with 10 μg compared to 5 μg, with a mean duration of 260.69 minutes in the 5 μg group and 362.30 minutes in the 10 μg group. Regarding pain scores at the 4th and 6th postoperative hours, the 10 μg group had lower pain scores compared to the 5 μg group. Singh AP et al., also found similar results, with a mean duration of analgesia of 200.0 minutes in the 5 μg group and 412.7 minutes in the 10 μg group, which was statistically highly significant [24]. The mean pain score was also significantly lower in the 10 μg group after 105 minutes.
In the current study, there were no side effects in either group. Similarly, in the studies conducted by Srikanth I et al., and Singh AP et al., the incidence of complications was comparable between the 5 μg and 10 μg groups [10,24].
Limitation(s)
The present study was conducted at a single centre and involved the same ethnic group, which limits its generalisability. It also lacks a comparison with a placebo group. All the patients were classified as ASA grade I and II; therefore, the safety and efficacy of the drugs on patients with ASA grade III or higher, those with cardiovascular co-morbidities, and patients over the age of 60 years were not studied.
Conclusion(s)
Intrathecal bupivacaine with dexmedetomidine at a dose of 10 μg causes an earlier onset and longer duration of sensory block, as well as providing a longer duration of analgesia compared to 5 μg dexmedetomidine. The 10 μg dexmedetomidine also hastens the onset of motor block and prolongs the duration of motor block. Both doses are haemodynamically safe without any significant side effects. In conclusion, 10 μg dexmedetomidine is superior to 5 μg dexmedetomidine as an adjuvant to hyperbaric bupivacaine in pelvic and lower limb orthopaedic surgeries.