A CSD (isthmocele) is formed due to defective healing of the caesarean scar. It is a wedge-shaped myometrial indentation at the site of a caesarean section. The incidence of this rare condition is increasing, almost paralleling the rise in the rate of caesarean section deliveries [1]. As per the current data, the reported prevalence of scar defects ranges from 24-70% [2-4]. However, this may be just the tip of the iceberg, as the condition may remain asymptomatic in many women. Approximately only 30% will present with symptoms [3].
In the last two decades, there has been increasing awareness of long-term gynaecological problems after caesarean deliveries, such as chronic pelvic pain, dyspareunia, dysmenorrhoea, postmenstrual spotting, menorrhagia, and even infertility [2,3,5], collectively referred to as caesarean scar syndrome [6].
Moreover, long-term obstetric sequelae are also on the rise, manifesting as a spectrum of disorders that begin with CSEP and placenta accreta. Caesarean scar pregnancy and early placenta accreta have been shown to share common histology, which means that this condition can progress to life-threatening obstetric complications of varying degrees, including placenta accreta associated with significant maternal morbidity and even mortality [7].
The wide availability of imaging facilities has facilitated the early diagnosis of this condition and its related complications. Unfortunately, there are still no standardised guidelines for its management. More than 30 treatment regimens have been described for the treatment of CSEP [8]. The Society for Maternal-Foetal Medicine (SMFM), after comparing various treatment modalities, has published recommendations regarding various diagnostic and treatment modalities for CSEP [9].
Management options range from various termination methods, including systemic or intrasac administration of methotrexate or KCL, to conservative methods like High-intensity Focused Ultrasound (HIFU), embolisation or double balloon catheter, hysteroscopic-guided removal of the CSEP after methotrexate, or laparoscopic excision of the sacs followed by scar repair. These options depend on clinical presentation, available facilities and surgical expertise [9].
So far, no single modality of treatment has been found to be outright superior to the others or applicable to all cases in the absence of gold-standard treatment guidelines for managing this ever-increasing, life-threatening obstetric and distressing gynaecological condition. Therefore, the present study was conducted with the aim of describing the demographic characteristics, clinical presentation, management, and outcomes in patients with CSD.
Materials and Methods
This was a retrospective cohort study conducted in the Department of Gynaecology, Gastrocare Hospital (tertiary care centre), Bhopal, Madhya Pradesh, India, from February 2020 to February 2023. Records of these patients were reviewed after obtaining permission from the Institutional head, as well as, the head of the hospital records section. Since it was an observational study requiring the review of records, ethical clearance from the district ethical board was not necessary.
Inclusion criteria: Case records of all women with a TVS-confirmed diagnosis of CSEP or CSD were included in the study.
Exclusion criteria: Case records of patients diagnosed preoperatively as CSEP or CSD but not confirmed intraoperatively were excluded from the study.
Records of 43 patients who presented with scar defects were reviewed. Demographic details, clinical presentations, prior treatments taken for the same condition, the diagnostic interventions used, the surgical procedures performed, and outcomes were recorded and analysed. TVS-based grading of scar defects was also done based on the study conducted by van der Voet LF et al., [3].
Statistical Analysis
Descriptive statistics, including frequencies, means and standard deviations, were calculated for demographic and clinical characteristics, prior treatments, diagnostic findings, and patient outcomes. Statistical analysis was conducted using SPSS software version 29.0.
Results
The majority of the women were in the age group of 30-40 years, and most had a history of one caesarean section. Out of the 11 women who presented with gynaecological disorders, six presented with postmenstrual spotting, two had menometrorrhagia, and three were undergoing evaluation for secondary subfertility [Table/Fig-1].
Demographic details with presenting complaints (N=43).
| Parameters | n (%) |
|---|
| Age (years) |
| 20-30 | 12 (27.9) |
| 31-40 | 31 (72.1) |
| Number of previous caesarean |
| 1 | 26 (60.5) |
| ≥2 | 17 (39.5) |
| Presenting complaints |
| Diagnosed caesarean scar pregnancy | 06 (13.9) |
| Complicated scar pregnancy post evacuation | 26 (60.5) |
| Menstrual disorder or AUB | 08 (18.6) |
| Secondary subfertility | 03 (6.9) |
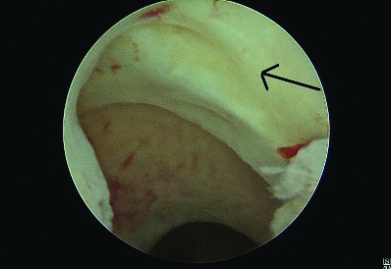
A total of eight patients with AUB underwent hysteroscopic resection of the distal edge of the scar defect [Table/Fig-2] using a resectoscope, followed by ball cauterisation of the base. At the three-month follow-up, they reported relief from their symptoms. Among the three patients with secondary subfertility who underwent diagnostic hysterolaparoscopy, the uterus was found to be acutely retroverted in two patients. Using hysteroscopic transillumination with Nirgianakis’ ‘Rendezvous technique’, the scar defect was identified by demonstrating a positive ‘Halloween sign’, and scar repair was performed laparoscopically in a double layer after excision of the fibrotic tissue [10]. Bilateral round ligament plication was performed for the correction of the retroverted uterus (Vervoot technique) [11]. One of these patients achieved successful conception five months after the procedure.
Hysteroscopic visualisation of scar niche.
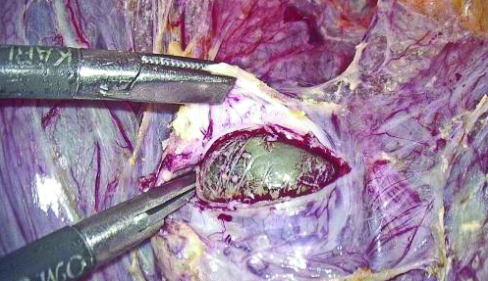
The CSEP is by far the most common sequelae of scar defects. Over this period, six patients were referred to the present study Institute as diagnosed cases of scar pregnancy [Table/Fig-3]. On TVS evaluation, they exhibited myometrial reduction of over 50% (RMT <3 mm). They were managed laparoscopically with excision of the scar pregnancy followed by double-layered closure. Round ligament plication was performed to correct retroversion in two of these cases [11]. One patient had heterotrophic bilateral tubal ectopic pregnancy along with CSEP, for which bilateral salpingectomy was performed alongside laparoscopic excision of CSEP.
Gestational sac in caesarean scar pregnancy.
Out of 43 patients assessed for CSDs using Ultrasound (USG) grading, 17 (39.5%) patients showed a myometrial reduction of more than 50% (RMT <3 mm), while 26 (60.5%) patients exhibited a myometrial reduction of less than 50% (RMT >3 mm) [Table/Fig-4].
TVS-based grading of scar defect (N=43).
| Grading of scar defect (USG grading) | n (%) |
|---|
| Myometrial reduction of >50% (RMT <3 mm) | 17 (39.5) |
| Myometrial reduction of >50% (RMT >3 mm) | 26 (60.5) |
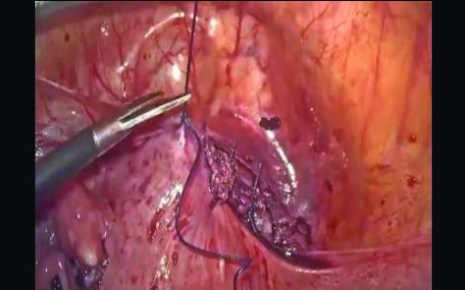
A total of 26 patients who had primarily undergone termination of pregnancy elsewhere were referred to the present study Institute with post-termination complications. Of these, 13 patients had taken medical abortion pills, five had received treatment with systemic methotrexate, and eight patients had undergone suction and evacuation. All of them experienced irregular bleeding post-termination of pregnancy. Upon evaluation by transvaginal scan, it was found that chorionic tissue was adherent at the scar site in all cases, with varying degrees of invagination of the chorionic tissue towards the bladder. The treatment plan was tailored based on the myometrial defect evaluated via transvaginal ultrasound and the preoperative radiological appearance of the tissue. Patients with myometrial reduction >50% (or RMT <3 mm) underwent laparoscopic resection of chorionic tissue and repair of the scar [Table/Fig-5]. Of these, two patients conceived and delivered at term by caesarean section. Patients with myometrial reduction <50% (or RMT >3 mm) were managed by hysteroscopic-guided excision of retained products.
Among the 43 patients evaluated, 32 (74.4%) patients were of CSEP and its complications, while 11 (25.6%) patients presented with gynecological problems. Of those managed laparoscopically, 14 (32.6%) patients were from the obstetric group, and 3 (6.9%) patients were from the gynaecological group. Hysteroscopic management was performed in 18 (41.8%) patients from the obstetric group and 8 (18.6%) patients from the gynaecological group [Table/Fig-6].
Modes of management (N=43).
| Mode of management | Obstetrics patients
{n=32 (74.4%)}, n (%) | Gynaecology patients
{n=11 (25.6%)}, n (%) |
|---|
| Laparoscopic approach | 14 (32.6) | 03 (6.9) |
| Hysteroscopic approach | 18 (41.8) | 08 (18.6) |
Discussion
Increasing caesarean section rates have led to a rise in complications associated with the procedure, such as the formation of CSD. However, Grechukhina O et al., reported a median of two prior caesarean deliveries among patients with the defect in their study [12]. Reported prevalence varies from 24-70% with TVS and from 56-84% with gel/saline instillation Sonohysterography (SHG) [2-4]. A literature review indicates that there may be an impairment of fertility in the presence of a defect, with the risk of reduced conception ranging from 4% to 19% [13,14].
Treatment for scar defects is currently indicated only in symptomatic women presenting with secondary infertility, previous scar ectopic pregnancies, recurrent miscarriage, AUB, and bothersome post-menstrual spotting or specific menstrual complaints. However, the case of heterotrophic pregnancy indicates that the presence of CSD alters the uterine and pelvic milieu in a manner that not only attracts the fertilised ovum for abnormal placentation but also likely renders the uterine cavity unwelcoming to the embryo, increasing the chances of tubal ectopic pregnancy. This further emphasises the need to diagnose and repair CSD even in the non pregnant state, thereby saving these women from various gynaecological morbidities, as well as, from the potentially deadly complication of caesarean scar pregnancy [15].
The surgical approach can be stratified based on the RMT. An RMT of atleast 3 mm is required for hysteroscopic resection, with a range of 2.5-4 mm reported in the literature to reduce the risk of bladder injury [3,16]. Laparoscopy is the preferred surgical approach for the repair of CSD, particularly if the residual myometrium is less than 3 mm thick and future fertility is desired [17,18].
In addition to laparoscopy, hysteroscopy-guided transvaginal repair is another approach to repair CSD. Luo L et al., reported the results of 42 patients who were managed through a pure transvaginal approach, achieving a clinical success rate for AUB of up to 92.9% (39 of 42 patients) [19]. Hysteroscopy-guided natural orifice repair of CSD could be a feasible alternative to laparoscopy-only procedures, but the authors did not perform this procedure [20].
Despite the recommendations given by the SMFM, treatment for CSEP remains a dilemma, with considerations on whether to offer medical management, choose a surgical approach, or use a combination of both. The use of Methotrexate (MTX) and other medical methods provides a treatment option for stable patients who wish to preserve their reproductive ability. Deb S et al., suggested that medical treatment with methotrexate can be considered for CSEP with a gestational age of less than seven weeks, a Beta Human Chorionic Gonadotropin (β-HCG) level <5,000 IU/L, a mass diameter <25 mm, no cardiac activity of the embryo, and the presence of myometrium between the gestational sac and bladder wall [21].
The recommendations by the SMFM Consult Series No. 63 also state that systemic methotrexate alone should not be used to treat CSEP (Grade 1C), and intragestational methotrexate can be used with or without other treatment modalities (Grade 2C) [9]. Unfortunately, in patients with CSEP who have been treated medically, the gestational mass can take weeks to months to resolve. After local conservative CSEP treatment involving 22 women, one study reported a mean time to resolution of 88 days (range: 26 to 77 days) [22]. Cheng Q et al., compared various surgical modalities for CSEP: Laparoscopy-assisted Operative Hysteroscopy (LAOH), Uterine Artery Embolisation (UAE) followed by LAOH, Dilation and Curettage (D&C), and UAE followed by D&C, and found that LAOH±UAE showed a higher success rate for patients with CSEP [23]. The method of LAOH, originally inspired by Wang CJ et al., aimed to minimise bladder injury and provide a strategy for the minimally invasive management of haemorrhage and scar repair [24].
As a referral centre, the authors have mostly received cases of failed medical management by methotrexate. These were managed hysteroscopically and laparoscopically based on the residual myometrial thickness. It has been observed that in women undergoing medical management, β-HCG takes much longer to return to normal compared to surgical management. This implies that medical management is not a feasible option for women desiring further pregnancies. The SMFM Consult Series also suggests that operative resection (with transvaginal or laparoscopic approaches when possible) or ultrasound-guided uterine aspiration should be considered for the surgical management of CSEP, and that sharp curettage alone should be avoided (Grade 2C) [9].
The incidence of CSD is increasing, along with the resultant long-term sequelae. The surgical techniques of uterine incision closure appear to be the most important determinant in the causation of CSD. In situations where a caesarean scar harbours a defect, a TVS is reliable for diagnosis. However, the question that requires further validation by larger studies is whether all cases of diagnosed CSD, desiring fertility, should undergo surgical correction to restore the uterine milieu.
Limitation(s)
The present is limited by a small sample size, which restricts the generalisability of the study findings. Consequently, the results may not be applicable to larger populations. Larger studies or multicentric meta-analyses are recommended to validate our findings. Additionally, the lack of standardised guidelines and criteria for the management of CSD and CSEP poses a challenge for practitioners in selecting the optimal treatment approach for this increasingly common pathology.
Conclusion(s)
The TVS is an effective tool for diagnosing CSD and CSEP. Surgical management can be guided by the RMT as determined by TVS. For cases with RMT greater than 3 mm, hysteroscopic management of CSD appears feasible. However, for RMT less than 3 mm, a laparoscopic approach with revision and repair of the caesarean scar is advisable. Further research is required to determine whether surgical correction of asymptomatic CSD is beneficial for women desiring future pregnancies.