Morganella Morganii- A case of an Emerging Hurdle in the Journey of Septic Wound Healing
Bhavna Pate1, Supriya Meshram2, Gargi Mudey3
1 Junior Resident, Department of Microbiology, Jawaharlal Nehru Medical College, DMIHER, Sawangi, Meghe, Wardha, Maharashtra, India.
2 Associate Professor, Department of Microbiology, Jawaharlal Nehru Medical College, DMIHER, Sawangi, Meghe, Wardha, Maharashtra, India.
3 Professor and Head, Department of Microbiology, Jawaharlal Nehru Medical College, DMIHER, Sawangi, Meghe, Wardha, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Bhavna Pate, Junior Resident, Department of Microbiology, Jawaharlal Nehru Medical College, DMIHER, Sawangi, Meghe, Wardha-442107, Maharashtra, India.
E-mail: bhavnaspate@gmail.com
Morganella morganii is a facultative gram-negative anaerobe present in the human gastrointestinal system as normal flora. Clinically, the organism is significant when it manifests as an opportunistic pathogenic infection elsewhere in the body. Morganella morganii (M. morganii) is a gram-negative bacillus found in the environment and among normal human intestinal flora. It is a well-known cause of urinary tract infections, wound infections, sepsis and other extraintestinal infections. It is also considered an opportunistic pathogen and has been known to occur in both community-acquired and nosocomial infections. The present case report depicts the outcome of wound healing in a 65-year-old, non diabetic, non hypertension male who had a history of a life-threatening necrotising soft-tissue infection in the right lower limb, which resulted in an infected ulcer due to M. morganii as the responsible pathogen. After proper diagnosis of the aetiologic pathogen as M. morganii, following pus and tissue culture and sensitivity tests from the infected wound, and after appropriate management with local wound care and antibiotics, there was early recovery, less morbidity, and an improved quality of life.
Anaerobic infection, Cellulitis, Debridement, Drug resistance
Case Report
A 65-year-old male presented to the Outpatient Department with a chief complaint of a non healing ulcer on the right foot, extending over the ankle for a period of one year. The patient had been treated by a local practitioner with local wound care, along with intermittent and irregular oral medications for a necrotising soft-tissue infection. The patient was non diabetic, non hypertension, and had no other co-morbid conditions. There was no significant history suggestive of arterial or venous insufficiency. The patient had a surgical history of cellulitis debridement of the same foot three years ago, with no other associated co-morbidities. There was no significant family history.
Local examination revealed an irregular ulcer with approximate dimensions of 25 cm by 10 cm, located on the dorsum of the foot and extending over the ankle region [Table/Fig-1a,b].
Pretreatment clinical photograph of right foot: a) Pretreatment; b) Post-treatment initiation.
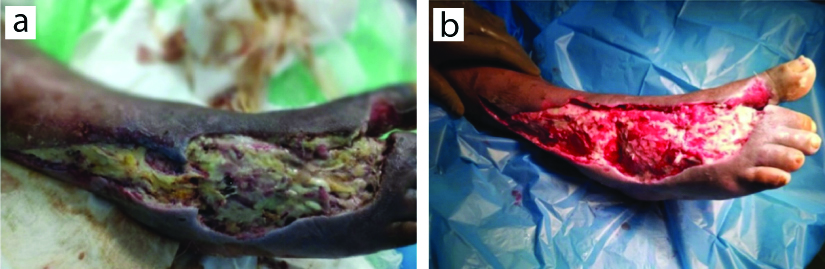
The ulcer floor was covered with slough and had a foul-smelling purulent discharge. Upon examination, the patient’s vitals were stable. Biochemical parameters indicated a hemoglobin level of 9.6 g/dL and elevated C-reactive Protein (CRP). The rest of the parameters were within normal limits [Table/Fig-2].
Blood investigation profile of the patient.
| Parameter | Patient value | Normal range |
|---|
| Haemoglobin | 9.6 g/dL | 14 to 18 g/dL (male) |
| CRP | 56 mg/dL | <0.3 mg/dL |
| MCHC | 32.6 g/dL | 32 to 36 g/dL |
| MCV | 83.1 fL | 80-100 fL |
| MCH | 27.1 pg/cell | 27-31 pg/cell |
| Total RBC count | 3.52 million cells/μL | 4.35-5.65 million cells/μL (male) |
| Total WBC count | 6400 cells/μL | 4,500-11,000 cells/μL |
| Total platelet count | 2.23 lakh cells/μL | 1.5-4 lakh cells/μL |
| HCT | 29.3% | 41% to 50% (male) |
| Monocytes | 4% of total WBC count | 2-8% of total WBC count |
| Granulocytes | 60 cells/μL | 1500-8500 cells/μL |
| Eosinophils | 02 cells/μL | 0-450 cells/μL |
| Basophils | 00 cells/μL | 0-200 cells/μL |
| Lymphocytes | 34 cells/μL | 1000-4800 cells/μL |
| RDW | 14.3% | 12-15% |
MCV: Mean corpuscular volume; MCH: Mean corpuscular haemoglobin; MCHC: Mean corpuscular haemoglobin concentration; RBC: Red blood cells; WBC: White blood cells; HCT: Haematocrit; RDW: Red cell distribution width
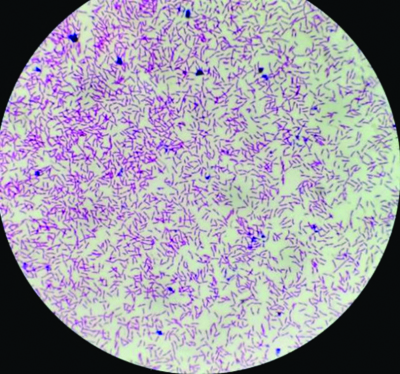
Radiological imaging of the right foot suggested no bony abnormality. The arterial and venous Doppler of the right lower limb was normal. The patient was hemodynamically stable, with no other systemic manifestations. The final diagnosis concluded a traumatic ulcer over the right lower limb. A wound swab from the patient was sent for a culture report, which suspected M. morganii [Table/Fig-3].
Gram staining image of the wound swab showing gram-negative Morganella morganii (H&E, 40x).
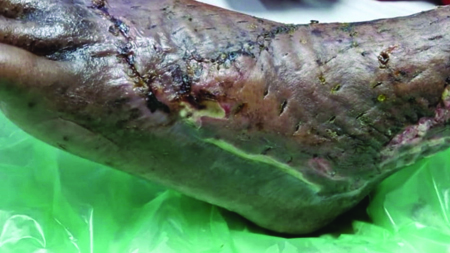
The final causative agent was reported as M. morganii infection by the VITEK® system. The antibiotic sensitivity profile indicated that the sample isolate was sensitive to ceftazidime, imipenem and amikacin, as determined by the Kirby-Bauer disk diffusion method. The drug sensitivity pattern of the sample was confirmed via the VITEK® machine before the initiation of medical management. The patient was started on injection ceftazidime (1 gm intravenous, three times a day for seven days) and injection amikacin (750 mg once daily for seven days). Along with the systemic therapy, local care of the wound was carried out by debridement at regular intervals, along with supportive care. After seven days, tablet cefpodoxime proxetil was started at a dose of 200 mg orally (twice daily) for 14 days. The patient responded well to the management regime and was found to be recovering well. After local infection control of the wound, the patient was subjected to negative pressure wound therapy, which further aided in recovery [Table/Fig-4].
Follow-up image of treated wound.
Discussion
Morganella morganii (M. morganii), also known as Proteus morganii, is a part of the common human microbiota. It is a rod-shaped, gram-negative facultative anaerobe. It has been reported as a cause of nosocomial infections, especially in postoperative recovery, where patients are vulnerable. M. morganii has been noted as a potentially fatal systemic infection in patients with compromised immune systems and in children [1].
Morganella morganii has been reported to have developed resistance against antibiotics such as penicillin and cephalosporin, which is attributed to the blaAmpC gene [2,3]. It is usually isolated from urinary tracts and wounds of patients with nosocomial infections. In recent times, infections caused by M. morganii have been on the rise in the skin and soft-tissues, making it a common infectious agent for bloodstream infections. Skin and soft-tissues have been observed as the most common infection foci [3-5].
M. morganii infections of the bloodstream have also included polymicrobial infections, which are mainly associated with Klebsiella pneumoniae, Proteus mirabilis, Escherichia coli, and Pseudomonas aeruginosa [3].
Antibiotic resistance has been observed to increase, and it has been termed the next ‘superbug’ [6]. M. morganii has been studied extensively for antibiotic resistance and some genetic elements such as mcr, MMG1-4, dfrA15, dfrA24, Tn7376, tetA, bla variants, and others [3,7-9]. M. morganii has also been reported as the main causative agent of wound infections and necrosis. It has been reported in patients with snake bites, as well [2,3,10]. A similar observation was noted in the present case, where the wound swab reported the presence of M. morganii and Pseudomonas species. It is identified by various methods, including the study of colony morphology, VITEK® systems, biochemical tests and molecular characterisation [2].
Although different treatment modalities are available, multidrug resistance has been observed in M. morganii. This is concerning, as despite therapy, mortality is reported in 15% of the patients [2,5]. M. morganii is also observed as a major contributor to neonatal sepsis in newborns [1]. Infections caused by M. morganii have been reported to be associated with severe complications such as septic arthritis [11] and have been noted to cause rapid progression of inflammation [6,9,12]. A research study involving 75 patients showed complete resistance to cefazolin, cefuroxime and nitrofurantoin, with a majority (95%) resistant to amoxicillin/clavulanic acid. Higher sensitivity was observed for drugs such as amikacin, gentamycin, ceftazidime and ceftriaxone [12]. A similar management approach using amikacin and ceftazidime was employed for this patient, which was found to be effective.
Drug-resistant M. morganii infections are common in diabetic patients, with foot ulcers being a frequent observation; however, such infections are reported rarely in non diabetic patients. Various novel approaches have been reported for the treatment of non healing ulcers in diabetic patients [13], but there is a scarcity of published data on the same in non diabetic patients. The prognosis for these types of individual or polymicrobial infections is poor in diabetic patients compared to non diabetic patients. The present case report serves as an example of that. M. morganii infections, if treated timely and adequately, leads to favourable prognostic outcomes.
Conclusion(s)
Morganella morganii infections have been on the rise, and there is a crucial need to monitor the incidence and resistance profile in non diabetic patients, which can aid clinicians in terms of incidence and management approaches. Timely diagnosis and drug sensitivity/resistance profiles can be helpful in the initiation of treatment and benefit the patient with a faster recovery. Future research and additional aetiological data are needed to combat this issue in a timely manner to prevent irreversible loss to the patient.
MCV: Mean corpuscular volume; MCH: Mean corpuscular haemoglobin; MCHC: Mean corpuscular haemoglobin concentration; RBC: Red blood cells; WBC: White blood cells; HCT: Haematocrit; RDW: Red cell distribution width
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jun 26, 2024
Manual Googling: Nov 28, 2024
iThenticate Software: Dec 30, 2024 (8%)
[1]. Chang HY, Wang SM, Chiu NC, Chung HY, Wang HK, Neonatal Morganella morganii sepsis: A case report and review of the literaturePediatr Int 2011 53(1):121-23.10.1111/j.1442-200X.2010.03241.x21342342 [Google Scholar] [CrossRef] [PubMed]
[2]. Zaric RZ, Jankovic S, Zaric M, Milosavljevic M, Stojadinovic M, Pejcic A, Antimicrobial treatment of Morganella morganii invasive infections: Systematic reviewIndian J Med Microbiol 2021 39(4):404-12.10.1016/j.ijmmb.2021.06.00534193353 [Google Scholar] [CrossRef] [PubMed]
[3]. Laupland KB, Paterson DL, Edwards F, Stewart AG, Harris PNA, Morganella morganii, an emerging cause of bloodstream infectionsMicrobiol Spectr 2022 10(3):e00569-22.10.1128/spectrum.00569-2235467403PMC9241912 [Google Scholar] [CrossRef] [PubMed]
[4]. Falagas ME, Kavvadia PK, Mantadakis E, Kofteridis DP, Bliziotis IA, Saloustros E, Morganella morganii infections in a general tertiary hospitalInfection 2006 34(6):315-21.10.1007/s15010-006-6682-317180585 [Google Scholar] [CrossRef] [PubMed]
[5]. Schultz E, Barraud O, Madec JY, Haenni M, Cloeckaert A, Ploy MC, Multidrug resistance Salmonella genomic island 1 in a Morganella morganii subsp. Morganii human clinical isolate from FrancemSphere 2017 2(2):e00118-17.10.1128/msphere.00118-1728435889PMC5397566 [Google Scholar] [CrossRef] [PubMed]
[6]. Bandy A, Ringing bells: Morganella morganii fights for recognitionPublic Health 2020 182:45-50.10.1016/j.puhe.2020.01.01632169625 [Google Scholar] [CrossRef] [PubMed]
[7]. Hassan J, Mann D, Li S, Deng X, Kassem II, First report of the mobile colistin resistance gene mcr-9.1 in Morganella morganii isolated from sewage in Georgia, USAJ Glob Antimicrob Resist 2022 29:540-41.10.1016/j.jgar.2021.11.01334896654 [Google Scholar] [CrossRef] [PubMed]
[8]. Luo XW, Liu PY, Miao QQ, Han RJ, Wu H, Liu JH, Multidrug resistance genes carried by a novel transposon Tn 7376 and a genomic island named MMGI-4 in a pathogenic Morganella morganii isolateMicrobiol Spectr 2022 10(3):e002652210.1128/spectrum.00265-2235510850PMC9241818 [Google Scholar] [CrossRef] [PubMed]
[9]. Lin TY, Chan MC, Yang YS, Lee Y, Yeh KM, Lin JC, Clinical manifestations and prognostic factors of Morganella morganii bacteremiaEur J Clin Microbiol Infect Dis 2015 34(2):231-36.10.1007/s10096-014-2222-825107625 [Google Scholar] [CrossRef] [PubMed]
[10]. Ngo ND, Le QX, Pham AQ, Nguyen NT, Ha HT, Dinh MMQ, Clinical features, bacteriology, and antibiotic treatment among patients with presumed Najabites in VietnamWilderness Environ Med 2020 31(2):151-56.10.1016/j.wem.2020.01.00232335010 [Google Scholar] [CrossRef] [PubMed]
[11]. Cetin M, Ocak S, Kuvandik G, Aslan B, Temiz M, Aslan A, Morganella morganii-associated arthritis in a diabetic patientAdv Ther 2008 25(3):240-44.10.1007/s12325-008-0026-x18327547 [Google Scholar] [CrossRef] [PubMed]
[12]. Alsaadi A, Alghamdi AA, Akkielah L, Alanazi M, Alghamdi S, Abanamy H, Epidemiology and clinical characteristics of Morganella morganii infections: A multicenter retrospective studyJ Infect Public Health 2024 17(3):430-34.10.1016/j.jiph.2023.12.01338262080 [Google Scholar] [CrossRef] [PubMed]
[13]. Abedi AS, McElroy JL, Valencia V, Worcester RM, Yu ZJ, Treatment of Morganella morganii-associated non-healing diabetic foot ulcer with vaporous hyperoxia therapy: A case reportCureus 2024 16(5):e6041310.7759/cureus.60413 [Google Scholar] [CrossRef]