Deep Vein Thrombosis and Pulmonary Embolism Secondary to Thrombophilic Disorder: A Case Report
Satbir Kaur Malik1, Vineetha Naga Lakshmi Giduturi2, Sindhuri Goud Nimmala3, Vijayashree Gokhale4
1 Assistant Professor, Department of Internal Medicine, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Dr. D. Y. Patil Vidyapeeth, Pune, Maharashtra, India.
2 Junior Resident, Department of Internal Medicine, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Dr. D. Y. Patil Vidyapeeth, Pune, Maharashtra, India.
3 Junior Resident, Department of Internal Medicine, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Dr. D. Y. Patil Vidyapeeth, Pune, Maharashtra, India.
4 Professor, Department of Internal Medicine, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Dr. D. Y. Patil Vidyapeeth, Pune, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Vineetha Naga Lakshmi Giduturi, B-49, Old Girls Hostel, Dr. D. Y. Patil Medical College and Hospital, Sant Tukaram Nagar, Pimpri, Pune-411018, Maharashtra, India.
E-mail: vineetha16giduturi@gmail.com
Iron Deficiency Anaemia (IDA) is a prevalent nutritional deficiency and a common haematological disorder characterised by low iron levels, which lead to reduced haemoglobin production, fatigue, pallor and diminished oxygen delivery. IDA increases the risk of venous thromboembolism by causing thrombocytosis, venous stasis and hypercoagulability. Although iron deficiency is a rare risk factor, recognising it as a significant risk factor can help prevent pulmonary thromboembolism if detected early and treated. The most common inherited thrombophilias—such as Factor V Leiden mutation, prothrombin G20210A mutation and deficiencies in protein C, protein S and antithrombin III—are a group of genetic conditions that predispose individuals to thrombotic events by influencing various factors involved in the coagulation cascade. Deep Vein Thrombosis (DVT) is a common condition that is part of venous thromboembolism disorders and represents the third most common cause of death from cardiovascular disease, following heart attacks and strokes. Authors hereby, report a rare and complicated case involving a 44-year-old male patient who experienced Pulmonary Embolism (PE), DVT, IDA and underlying abnormalities in two key anticoagulant proteins: Protein C and Protein S. Anticoagulation with low molecular weight heparin was initiated to treat the PE and DVT. Based on the patient’s thrombophilia profile, a newer oral anticoagulant, rivaroxaban, was prescribed as a long-term anticoagulant.
Iron deficiency anaemia, Protein C deficiency, Protein S deficiency, Rivaroxaban, Thrombophilia profile
Case Report
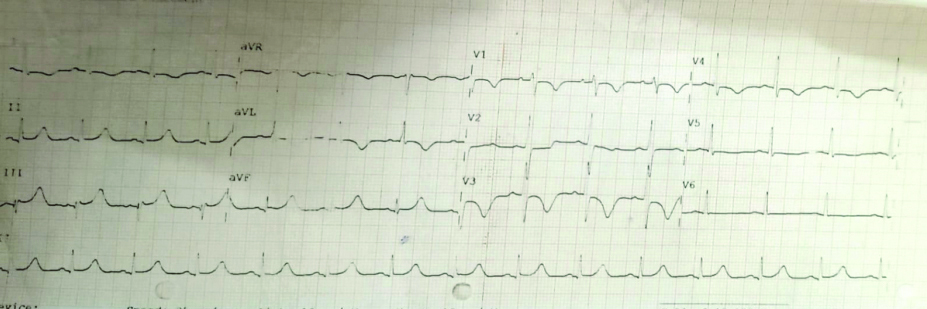
A 44-year-old male who works as a truck driver arrived at the emergency room complaining of exhaustion, dyspnoea, discomfort and swelling in his left leg that had been present for one to two days. The symptoms started suddenly and worsened over time; there was no accompanying sweating, palpitations, or chest pain. In the last one to two months, the patient reported experiencing exertional dyspnoea, classified as New York Heart Association (NYHA) Grade 2. Examination results showed a consistent pulse rate of 120 beats per minute, a blood pressure reading of 90/60 mmHg and a saturation of 94% on room air. Pallor was present [Table/Fig-1]. A systolic murmur in the tricuspid area was noted on auscultation during the systemic examination. Electrocardiography (ECG) showed T wave inversions in leads II, III, avf, V4, V5 and V6, suggestive of S1Q3T3 [Table/Fig-2].
Showing pallor in the lower palpebral conjunctiva.
Showing ECG with S1Q3T3 pattern and T wave inversion in lead II, III, v4, v5 and v6 suggestive of pulmonary thromboembolism.
Laboratory studies revealed a haemoglobin level of 7.4 g/dL, a Total Leukocyte Count (TLC) of 6700 and a platelet count of 300,000 (N/L/M-61/29/8). Serum iron levels were 8 micrograms/dL, serum ferritin levels were 5.67 nanograms per millilitre, total iron binding capacity was 404 micrograms per decilitre and serum transferrin saturation was 19.8%. Serology yielded negative results. The D-dimer was 1500 nanograms per millilitre (elevated). The thrombophilia profile indicated Protein C activity at 31%, Protein S activity at 60%, antithrombin III activity at 78% and activated protein C resistance at 161.60 seconds, suggestive of Protein C and Protein S deficiency. Occult blood tests and routine stool microscopy yielded negative results. Upper endoscopy revealed duodenal fissuring.
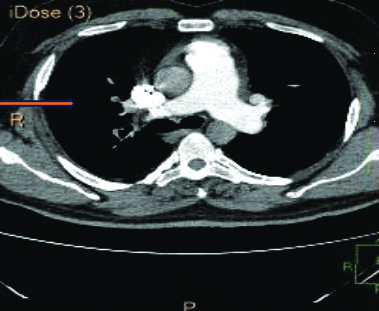
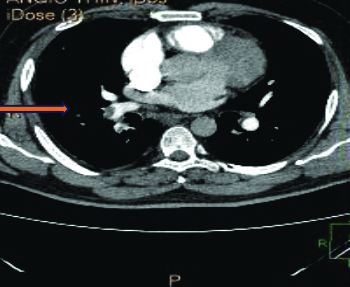
A 2-Dimensional Echocardiography finding indicated severe tricuspid regurgitation, pulmonary arterial hypertension and dilatation of the right atrium and ventricle. A Computed Tomography (CT) scan of the chest and pulmonary artery [Table/Fig-3,4] revealed a large filling defect causing near-total occlusion of the right pulmonary artery, extending into the truncus anterior and interlobar pulmonary artery and affecting all basal segmental arteries. The above findings are suggestive of pulmonary thromboembolism.
Showing CT scan of chest and pulmonary artery showing filling defect and occlusion in right pulmonary artery.
Showing CT scan of chest and pulmonary artery showing a filling defect in right pulmonary artery with contrast seen distal to the occlusion.
Lower limb venous duplex showed thrombosis in the bilateral lower limbs. The patient received intravenous iron injections as part of their treatment. Following this, they received ten injections of enoxaparin (0.6 units subcutaneously twice daily) and 15 mg twice daily of rivaroxaban for anticoagulation. After anticoagulation, the swelling in the left leg subsided and the patient is now receiving routine follow-up care. On follow-up, the patient is vitally and clinically stable and is continuing to take tablet rivaroxaban 15 mg twice daily.
Discussion
Normal iron levels act as antioxidants and decrease thrombopoiesis. Higher platelet counts, thrombocytosis and venous stasis are risk factors linked to iron deficiency anaemia, which increases the risk of thromboembolism in both the venous and arterial systems. The patient experienced PE secondary to DVT and had deficiencies in protein C and S. A study by Chi G et al., suggested that decreased iron levels, along with venous stasis, contribute to venous thromboembolism, which the authors also observed in their report [1]. The patient has been on anticoagulation since admission and has symptomatically improved. Blood clots can form in the body’s deep veins, primarily in the legs, due to a serious medical condition called DVT. These blood clots can break off and travel to the lungs, where they can cause PE, a potentially lethal condition [2].
In a study by Noiri JI et al., it has been observed that deficits in protein C and protein S are genetic conditions that increase the risk of blood clots and can sometimes co-exist in individuals with DVT, similar to the case described here [3]. The body’s clotting process is aided by natural anticoagulants like protein C and protein S. A deficiency in these proteins impairs the body’s ability to prevent blood clots from forming, which raises the risk of clotting diseases such as PE and DVT [4]. A patient with DVT also has deficits in protein C and protein S. To avoid complications like PE and prevent blood clots from recurring, healthcare professionals must closely monitor and manage the patient’s condition [5].
Due to the uncommon combination of IDA, DVT, PE and deficits in protein C and protein S, a multidisciplinary team of haematologists, pulmonologists and vascular surgeons was required to manage the patient’s condition. The need for therapy and management increases when a patient with DVT also has deficits in protein C and protein S. The patient’s unique combination of IDA, DVT, PE and deficits in protein C and protein S made controlling their illness extremely challenging and necessitated collaboration among specialists.
To prevent the formation of new blood clots and reduce the risk of clotting problems, treatment for individuals with DVT and protein C and S deficits may include a combination of anticoagulant drugs, such as warfarin or heparin, as observed in the study by Kearon C et al., [5,6]. Additionally, individuals may be advised to modify their lifestyles—such as maintaining a healthy weight, engaging in regular exercise and quitting smoking—to lower their risk of developing more blood clots [7].
In a study by Zhang N et al., it was shown that patients with protein C and protein S deficiencies and DVT must have regular follow-up appointments and monitoring to ensure their condition is well-managed and to adjust their treatment plan as necessary. Furthermore, patients should be made aware of the warning signs and symptoms of PE and blood clots so they can seek help promptly if they notice any alarming symptoms [8].
Conclusion(s)
A high index of suspicion for venous thromboembolism should be maintained if a patient presents with sudden onset of breathlessness. Protein C and S deficiencies further complicate such cases, as these inherited conditions predispose individuals to abnormal blood clotting. While anticoagulant therapy remains the cornerstone of treatment, genetic counselling and testing should be offered to family members to identify other individuals at risk and to guide management strategies. Overall, a multidisciplinary approach involving haematologists, cardiologists, pulmonologists and other specialists is essential in addressing the complex interplay of risk factors and tailoring individualised treatment plans for each patient. In the future, ongoing research and advancements in the field of thrombosis will hopefully lead to improved diagnostic techniques, more targeted therapies and better outcomes for patients.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 13, 2024
Manual Googling: Oct 14, 2024
iThenticate Software: Nov 02, 2024 (6%)
[1]. Chi G, Gibson CM, Hernandez AF, Hull RD, Kazmi SHA, Younes A, Association of anemia with venous thromboembolism in acutely ill hospitalized patients: An APEX trial substudyAm J Med 2018 131(8):972.e1-972.e7.10.1016/j.amjmed.2018.03.03129660351 [Google Scholar] [CrossRef] [PubMed]
[2]. Puri S, Grover AK, Narula AK, Choudhry PN, Gupta AP, Atypical presentation of pulmonary embolism and deep vein thrombosis due to protein S deficiency in a young female with chest painThe Egyptian Journal of Internal Medicine 2022 34(1):3710.1186/s43162-022-00124-w [Google Scholar] [CrossRef]
[3]. Noiri JI, Matsuzoe H, Nagaya S, Nishio R, Matsumoto D, Takaishi H, A case of venous thromboembolism caused by protein C deficiency due to a novel gene mutationJ Cardiol Cases 2022 26(5):360-63.10.1016/j.jccase.2022.07.01236312771PMC9606104 [Google Scholar] [CrossRef] [PubMed]
[4]. Middeldorp S, van Hylckama Vlieg A, Thrombophilia: From empiric evidence to pharmacogeneticsJ Thromb Haemost 2016 14(5):885-89.10.1111/jth.1328226854753PMC5540659 [Google Scholar] [CrossRef] [PubMed]
[5]. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Antithrombotic therapy for VTE disease. CHEST guideline and expert panel reportChest 2016 149(2):315-52.10.1016/j.chest.2015.11.02626867832 [Google Scholar] [CrossRef] [PubMed]
[6]. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S, Use of the direct oral anticoagulants in obese patients: Guidance from the SSC of the ISTHJ Thromb Haemost 2016 14(6):1308-13.10.1111/jth.1331026954307 [Google Scholar] [CrossRef] [PubMed]
[7]. Rivera-Lebron B, McDaniel M, Ahrar K, Alrifai A, Dudzinski DM, Fanola C, Diagnosis, treatment and follow up of acute pulmonary embolism: Consensus practice from the PERT consortiumClin Appl Thromb Hemost 2019 25:107602961985303710.1177/107602961985303731185730 [Google Scholar] [CrossRef] [PubMed]
[8]. Zhang N, Sun DK, Tian X, Zheng XY, Liu T, Protein C deficiency with venous and arterial thromboembolic eventsWorld J Clin Cases 2024 12(12):2000-03.10.12998/wjcc.v12.i12.200038680262PMC11045517 [Google Scholar] [CrossRef] [PubMed]