Introduction
In India, the reported incidence of eclampsia varies from 0.179 to 3.7% [1-3]. Maternal mortality among eclamptic women also shows significant variation, ranging from 2.2 to 23% [3-5]. Emergency Obstetric Hysterectomy (EOH) is a necessary operative procedure in many obstetric emergencies, including Placenta Accreta Spectrum (PAS) and Post Partum Hypertension (PPH), with an incidence of 0.504 per 100 deliveries [6]. Retained placenta complicates 2% of all deliveries and has a case mortality rate of nearly 10% in rural areas [7]. A study by Alemu A et al., found that 10.5% (95% CI: 6.8-15.7) of women with uterine rupture have died, with median recovery and death times of eight and three days, respectively, and Interquartile Ranges (IQR) of 7-11 days and 2-5 days [8]. Furthermore, ectopic pregnancy accounts for 6% of all pregnancy-related deaths and is the highest contributor to haemorrhage-related fatalities [9].
The present case series will delve into near-miss cases among women who experienced eclampsia, underwent subtotal hysterectomy with colpotomy, retained placenta, uterine rupture, and scar ectopic pregnancy. By examining these cases, author aimed to highlight the critical nature of timely medical interventions and the importance of improving maternal healthcare systems to prevent such life-threatening complications. This exploration will provide valuable insights into the clinical management and outcomes of these severe obstetric emergencies, contributing to the body of knowledge necessary for enhancing maternal health strategies and protocols.
Case Series
Case 1
Severe preeclampsia in a Primigravida with DCDA twins patient presentation: A 28-week primigravida, married for one and a half years and conceived spontaneously, initially presented to a primary health centre with bilateral pedal oedema and severe hypertension. Her blood pressure at the time was 170/110 mmHg. Consequently, she was referred to Hospital for further management.
Clinical examination: Upon presentation to hospital, the patient was in fair general condition and afebrile. Her blood pressure was 180/110 mmHg, indicating severe hypertension, and her pulse rate was 88 beats per minute (bpm). Clinical examination revealed bilateral pitting pedal oedema and abdominal wall oedema. Urine dipstick analysis showed albumin 4-plus. Deep Tendon Reflexes (DTR) were normal. Per abdominal examination indicated a uterine size corresponding to 32-34 weeks with palpable multiple foetal parts. Twin-A was in a longitudinal lie with vertex presentation and a Foetal Heart Rate (FHR) of 130 bpm, as recorded by Doppler. Twin-B was also in a longitudinal lie with vertex presentation but had no cardiac activity. Vaginal examination showed a minimally effaced cervix with a closed os. Foley catheterisation was performed, but there was no urine output.
Investigations: An emergency Ultrasound (USG) of the abdomen and pelvis showed intrauterine gestation of DCDA twins, with Twin-A in a longitudinal lie, vertex presentation, and an Estimated Foetal Weight (EFW) of 1418 grams. The FHR of Twin-A was 114 bpm, while Twin-B had no FHR. A notable drop in the FHR of Twin-A was observed. The placental location was anterior, with an Amniotic Fluid Index (AFI) of 10-11 cm for both twins. Additionally, a hypoechoic lesion measuring 12.5×4.6 mm posterior to the placenta was identified, raising concerns about a retroplacental haematoma. Colour Doppler imaging indicated a lack of blood flow in this area, and the mean uterine artery Pulsatility Index (PI) was 2.25, exceeding the 99th percentile.
Management: The patient was admitted to the obstetrics Intensive Care Unit (ICU). She was immediately started on intravenous (i.v.) labetalol at 20 mg stat, followed by an i.v. infusion at 2 mg/minute. An i.v. magnesium sulfate was administered according to the Pritchard regimen. Furosemide 40 mg (i.v.) was given stat due to anuria. An emergency LSCS was performed under general anaesthesia. Intraoperatively, 400 cc of blood clots were found in the uterus. Twin-A was delivered and immediately shifted to the Neonatal Intensive Care Unit (NICU). Twin-B was an intrauterine death.
Postsurgery, the patient was shifted to the obstetrics ICU. Her blood pressure was managed aggressively with multiple antihypertensives, including nitroglycerin infusion, prazosin, and dihydropyridine calcium channel blockers.
Postoperative complications and management: On Postoperative Day (POD)-0, a few hours after the LSCS, the patient exhibited active bleeding from the suture site. Repeat Haemoglobin (Hb) dropped to 4.8 g/dL, and the patient developed severe hypotension and a low Glasgow Coma Scale (GCS). She was intubated and placed on mechanical ventilator support. The profuse bleeding was attributed to DIC secondary to HELLP syndrome. Coagulation studies showed low serum fibrinogen and elevated Prothrombin Time- International Normalised Ratio (PT-INR), indicating coagulopathy. She received Fresh Frozen Plasma (FFP) and multiple packed cell transfusions to address severe anaemia secondary to massive blood loss. Despite best efforts, her hypotension was refractory to fluid replacement, necessitating inotropic support.
Urine output remained nil, suggesting acute tubular necrosis secondary to massive blood loss. Arterial blood gas analysis revealed severe metabolic acidosis with severe hyperkalemia. An Electrocardiogram (ECG) showed tall, peaked T-waves. Immediate anti-hyperkalemic measures included stat administration of i.v. calcium gluconate 10% (1000 mg per 10 mL) and a drip of 12 units of regular insulin in 100 mL of 25% dextrose over 20 minutes. A nephrology consultation was obtained, and the patient was initiated on Slow-efficiency Haemodialysis (SLED).
Her Complete Blood Counts (CBC), Renal Function Tests (RFT), and Liver Function Tests (LFT) were monitored daily. With timely haemodialysis and a multifaceted approach to sepsis prevention, her parameters gradually improved. Urine output increased progressively. She was extubated on POD twelve and later shifted to the general ward. Subsequently, the patient was discharged home in good health.
Case 2
Emergency subtotal obstetric hysterectomy in a 37-year-old G3P2L0A1 woman with 28.5 weeks of IVF-conceived DCDA twins patient presentation: A 37-year-old woman, gravida 3, para 2, living 0, abortus 1, at 28.5 weeks of gestation with DCDA twins conceived through IVF, presented with abdominal pain of one-day duration. She had a cervical cerclage (Shirodhkar’s method using mercelene tape) due to a short cervix, a history of metroplasty at the fundus and right lateral wall, hysteroscopic adhesiolysis on the left lateral wall, and rubella IgG positivity. She had been on daily subcutaneous enoxaparin 40 mg and oral aspirin 75 mg since embryo transfer. The patient had been admitted three times previously for vaginal bleeding and abdominal pain.
Course of labour: Upon admission, the patient’s per-abdominal examination showed uterine contractions 2/15-20’/10’, a cervix dilated 2-2.5 cm, absent membranes, and significant bleeding. An urgent USG revealed:
Twin A: cephalic presentation with adequate AFI
Twin B: transverse lie with adequate AFI
Given the history of previous LSCS and the presence of twin gestation in latent labour, an emergency LSCS was performed.
Postoperative course: After surgery, the patient was shifted to the ICU with an abdominal drain in-situ for close observation. Initial vitals were Blood Pressure (BP) 120/70 mmHg, Pulse Rate (PR) 126 bpm, and oxygen saturation 100% on room air. She was on adrenaline (2 mL/hr) and received 1 Packed Cell Volume (PCV) and 2 FFP transfusions. Despite continuous adrenaline support (8 mL/hr), her BP dropped to 90/60 mmHg, and her PR increased to 156 bpm. Two hours later, her uterine tone became intermittently flabby, BP dropped to 50/30 mmHg, and PR increased to 158 bpm. Minimal collection was observed in the abdominal drain.
Emergent intervention: The patient was started on a vasopressin infusion (1-2 mL/hr) and increased adrenaline (10 mL/hr). Despite these measures, the uterus remained intermittently flabby, and a vaginal pack with sponge holders was placed on the cervix. An USG revealed a hyperechoic collection inside the uterus with clots and minimal collection in the perihepatic and perisplenic spaces. Due to primary PPH, hypotension, tachycardia, a drop in Hb (6.3 g/dL), and USG findings, an emergency subtotal obstetric hysterectomy was performed.
Further management: Post-hysterectomy, the patient remained in the surgical ICU with an abdominal drain and Foley catheter. She required multiple transfusions {7 PCV, 6 FFP, 1 Single Donar Platelets (SDP)} over two days. Antibiotics (piperacillin-tazobactam, metronidazole, and amikacin) were administered. Despite the transfusions, her Hb remained low, and further blood tests showed elevated Total Leucocyte Count (TLC) and decreasing platelets. She developed a fever and burning micturition, necessitating further investigation and antibiotic adjustments.
Complications and additional procedures: Subsequent abdominal USG revealed pelvic collections, and Contrast-enhanced Computed Tomography (CECT) abdomen confirmed intra-peritoneal abscesses and a possible cutaneous draining sinus tract. An emergency colpotomy was performed on POD 17, and diagnostic aspiration on POD 2 of the colpotomy revealed a haematoma without abscess. The abdominal drain was removed on POD 3. The patient was discharged on the day of colpotomy and day 24 post-hysterectomy.
Neonatal outcome: Both twins had adverse outcomes:
Twin A (male) expired on day 8 due to cardiac arrest.
Twin B (female) expired on day 13 due to cardio-respiratory arrest, pulmonary haemorrhage, DIC, and Multiorgan Dysfunction Syndrome (MODS).
Case 3
Retained placenta in a 35-year-old primigravida at 39.4 weeks gestation patient presentation: A 35-year-old primigravida presented at 39.4 weeks of gestation (dated by first-trimester USG) in active labour. She had no history of leakage of amniotic fluid, vaginal bleeding, or significant past medical history. There were no previous surgeries related to the uterus. The patient reported good foetal movements, and a contraction stress test was reassuring.
Course of labour: Upon admission, the patient’s cervix was 3 cm dilated. Labour was augmented with 5 units of oxytocin. The patient progressed normally through labour, and after five hours, she delivered via normal spontaneous vaginal delivery. Post-delivery, gentle traction was applied for 15 minutes to deliver the placenta, but it did not separate. A continuous i.v. oxytocin drip {20 U in 500 mL Ringer’s Lactate (RL)} was administered, and the bladder was emptied intermittently. After 30 minutes of unsuccessful attempts, a tablet of 400 mcg of misoprostol was given sublingually, followed by an injection of 250 mcg of carboprost after an hour.
Given the risk of severe haemorrhage, the patient consented to a manual removal of the placenta in the Operating Theatre (OT), with the possibility of a hysterectomy. Consent was also obtained for the transfusion of blood and blood products. In the OT, after administering spinal anaesthesia, only 25% of the placental bits could be separated, with the rest being morbidly adherent. A sample was sent for histopathological examination, which confirmed retained placenta. One unit of PCV was transfused intraoperatively.
The procedure was abandoned due to the morbid adherence of the placenta and the absence of active bleeding. The patient was shifted to the ICU.
Postoperative course: The patient was stable in the ICU with minimal vaginal bleeding and was under close observation. She was started on i.v. antibiotics: injection of piperacillin-tazobactam 4.5 g TDS, injection of metronidazole 100 cc TDS, and injection of amikacin 250 mg BD for seven days. Tablet misoprostol 200 mcg was administered per rectum every four hours, totaling 1800 mcg. The uterus remained tonically contracted, and no active bleeding was noted.
On POD 2, a Complete Blood Count (CBC) revealed an Hb of 4.7 g/dL, a Total Leukocyte Count (TLC) of 17,100/mcL, and platelets of 193,000/mcL. Liver Function Tests (LFTs) and Renal Function Tests (RFTs) were within normal limits. The patient received three units of PCV and four units of FFP. Approximately 50 to 80 grams of blood clots were removed vaginally, and tablet misoprostol 600 mcg was administered per rectum. On day 3, 200 cc of blood clots were expelled vaginally, prompting a decision for exploration and examination under anaesthesia. In the OT, blood clots were removed, but the placenta remained adherent to the fundal wall. One unit of PCV was transfused intraoperatively. An USG showed a retained placenta in the fundus and multiple clots in the lower uterine body and cervix.
On POD 4, a tablet of methergine 0.25 mg was started thrice a day, and one tablet of mifepristone 200 mcg was given. CBC on POD 4 showed an Hb of 6.9 g/dL, TLC of 9,400/mcL, platelets of 162,000/mcL, and beta hCG of 650 mIU/mL. One unit of PCV was transfused. In total, seven units of PCV and four units of FFP were transfused.
On POD 6, an Magnetic Resonance Imaging (MRI) of the abdomen and pelvis revealed an adherent placenta in the fundal region (68×51×54 mm) and blood clots in the cervical canal. The lesion extended through the myometrium to the serosal surface, causing marked thinning of the adjoining uterine wall on the left side.
On POD 8, with an Hb of 8.9 g/dL, TLC of 15,800/mcL, and platelets of 181,000/mcL, the patient was shifted to the ward as she was vitally stable. On POD 9, the patient passed blood clots but had no active bleeding. On POD 10, the placenta was expelled spontaneously and completely. An USG confirmed an empty uterine cavity. Histopathological examination of the expelled placenta confirmed maternal floor placental infarction with chorioamnionitis. The patient was discharged on POD 15 with stable vitals.
Case 4
Uterine Rupture in a 32-year-old multigravida at 40.3 weeks gestation case presentation: The patient, a 32-year-old multigravida (G3P1L1A1) at 40.3 weeks of gestation, was referred from a private Hospital. Upon referral, her general condition was moderate, with a blood pressure of 90/60 mmHg, a pulse rate of 142 bpm, a Foley catheter in-situ with 100 mL of blood-stained urine, absent FHR, and intravenous fluids and haemaccel administered.
History: The patient reported experiencing abdominal pain and vaginal bleeding for one day. She had a history of spontaneous conception with two previous Full-term Normal Vaginal deliveries (FTNVDs) and one Medical Termination of Pregnancy (MTP) at 1.5 months of Amenorrhoea (moA) three years prior. Her last scan, conducted eight days ago, indicated a single live intrauterine gestation of 37.2 weeks with cephalic presentation, an Amniotic Fluid Index (AFI) of 11-12 cm, an anterior placenta, an Estimated Foetal Weight (EFW) of 3306 grams, and a single loop of cord around the neck.
Initial examination: Upon arrival, the patient was afebrile with a blood pressure of 110/60 mmHg, a pulse rate of 118 bpm, SpO2 of 100% on room air, and marked pallor. There were no premonitory symptoms; DTRs were normal, and urine albumin was 2+. The Foley catheter output included 200 mL of urine with significant haematuria. The cardiovascular, respiratory, and central nervous systems were normal.
Obstetric examination: Upon obstetric examination, the patient’s general condition was moderate. She was afebrile with a blood pressure of 110/60 mmHg, a pulse rate of 118 bpm, and SpO2 of 100% on room air. Marked pallor was noted, and urine albumin was 2+, with normal DTRs. The Foley catheter output showed 200 mL of urine with significant haematuria. The cardiovascular, respiratory, and central nervous systems were normal. The abdominal examination revealed a uterus of term size with no palpable contractions, although foetal parts were felt, and FHR was absent. A speculum examination showed no demonstrable leak, but active bleeding was observed. On vaginal examination, the cervix was 6-7 cm dilated, fully effaced, with a vertex presentation, absent membranes, and the foetal head positioned high.
Diagnosis and management: A clinical diagnosis of suspected uterine rupture and abruptio placentae was made. The patient was shifted for an USG, which suggested intrauterine foetal demise with intra-amniotic echoes/blood clots.
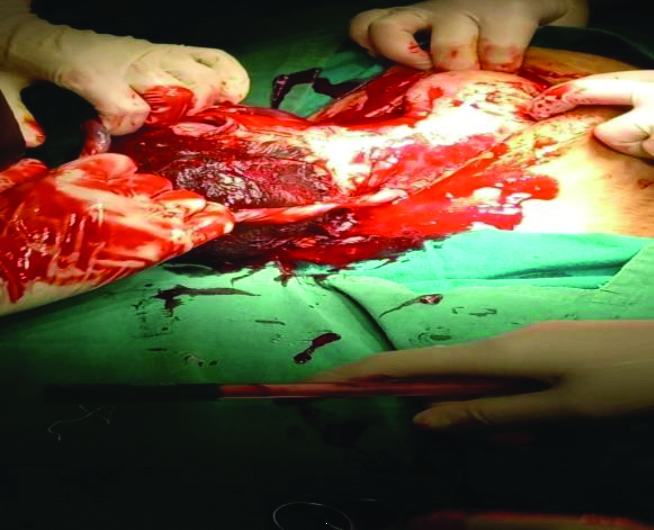
Operative findings and procedure: The patient was urgently taken to the Operating Theatre (OT) for an exploratory laparotomy. A midline vertical incision was made, and upon opening the abdomen in layers, a gush of blood was noted upon opening the visceral peritoneum. The foetus was found in the peritoneal cavity and delivered in a vertex presentation.
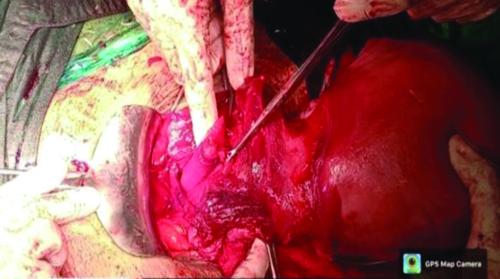
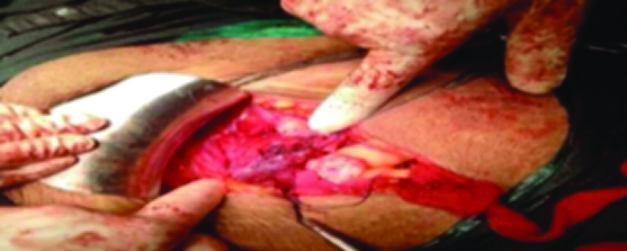
The lower uterine segment and anterior wall of the vagina showed a corrugated margin, indicating rupture [Table/Fig-1,2 and 3]. Given the extensive nature of the rupture, an obstetric hysterectomy was performed. The vaginal vault was closed in a continuously interrupted manner. The specimen was sent for histopathological examination.
Foetus in peritoneal cavity with vertex presentation.
Corrugated margin of lower uterine and anterior wall of vagina traced.
Vaginal vault closed in continuous interrupted manner.
Case 5
Scar ectopic pregnancy in a 27-year-old female patient presentation: A 27-year-old female, Gravida 4 Para 2 Living 2 Abortion 1 (G4P2L2A1) with a history of two previous LSCS, presented with two months of amenorrhoea and a positive urine pregnancy test at home. This was her first visit to Dr. D.Y. Patil Hospital for the current pregnancy, and her Last Menstrual Period (LMP) was on 11/05/2022, giving her a Period of Gestation (POG) of 11.2 weeks. She had no other complaints at the time of presentation.
Medical and obstetric history: The patient had her menarche at the age of 14 years and experienced regular menstrual cycles, with her LMP on 11/05/2022. She had been married for eight years in a non consanguineous marriage. Her past obstetric history included the birth of a male child seven years ago via LSCS due to oligohydramnios, with a birth weight of 2 kg (P1L1). Five years ago, she underwent a MTP at four months of amenorrhea due to a missed abortion, managed with Dilation and Evacuation (D&E) (A1). Four years ago, she gave birth to a female child via LSCS, which was performed due to her previous LSCS, and the child had a birth weight of 3.7 kg (P2L2). She was currently in her fourth pregnancy (G4). There were no significant past medical, personal, or family histories.
Gynaecological examination: On abdominal examination, the patient’s abdomen was soft and non tender, with no scar tenderness. During the pelvic examination, the per speculum inspection revealed a healthy cervix and vagina. The per vaginal examination showed that the uterus was of normal size, anteverted, with bilateral fornices that were free and non tender.
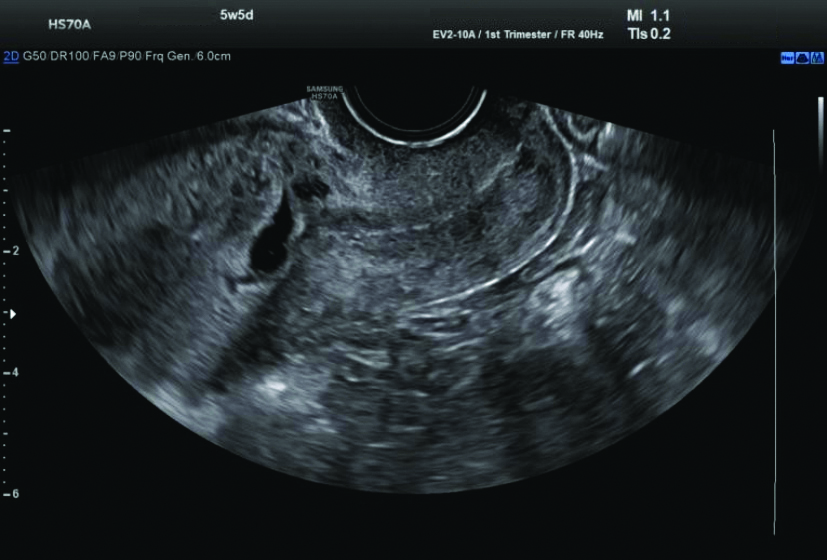
Ultrasound (USG) examination: The USG revealed a single gestational sac in the lower uterine segment at the region of the previous caesarean scar, extending into the anterior myometrial wall with surrounding increased vascularity and decidual reaction, suggestive of scar ectopic pregnancy. The foetal pole and yolk sac were not visualised. The mean gestational sac measured 9.9 mm, corresponding to 5.5 weeks [Table/Fig-4,5].
Ultrasound (USG) revealed a single gestational sac in the lower uterine segment.
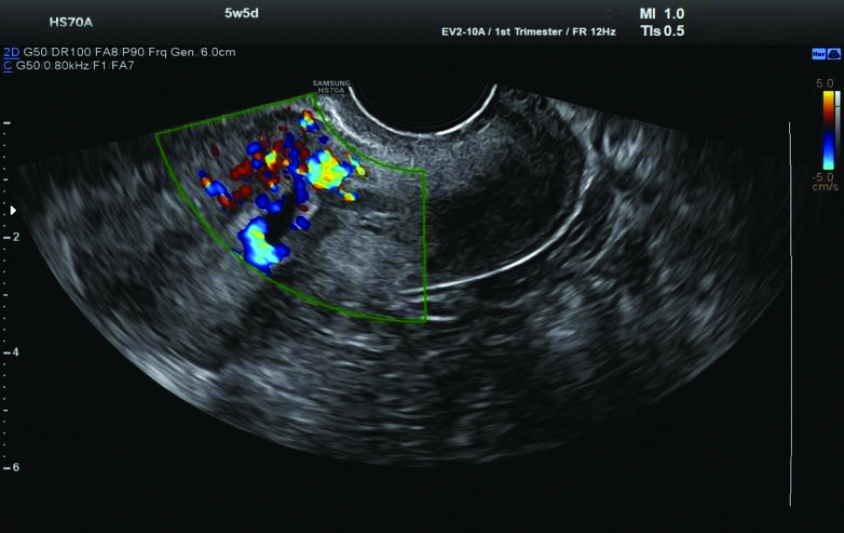
Increased vascularity and decidual reaction on Ultrasound (USG).
Course of treatment: All routine investigations were within normal limits. The patient was managed medically with a single dose of intramuscular methotrexate at 1 mg/kg body weight. Serial β-Human Chorionic Gonadotropin (hCG) monitoring was initiated, starting with an initial β-hCG level of 9725 mIU/mL. After 48 hours, the β-hCG level decreased to 5679 mIU/mL. Following another 48 hours, the level further dropped to 3364 mIU/mL. An additional 48 hours later, the β-hCG level was 2336 mIU/mL. On Day 10, the β-hCG level was 845 mIU/mL. The β-hCG levels showed a consistent decreasing trend, and the patient was discharged on Day 12 with a β-hCG value of 400 mIU/mL.
Discussion
The management of severe preeclampsia in a primigravida with DCDA twins underscores the need for vigilant monitoring and aggressive treatment to prevent maternal and foetal morbidity and mortality. Early identification of severe hypertension and proteinuria, coupled with the use of antihypertensive therapy and magnesium sulfate for seizure prophylaxis, is pivotal in stabilising the mother. In present case, the decision to proceed with an emergency caesarean section, despite the risk of preterm delivery, was critical due to the deteriorating foetal condition of Twin A and the intrauterine demise of Twin B. This approach highlights the importance of proactive management in severe preeclampsia, especially in resource-limited settings. Han Q et al., have shown that the probability of twin pregnancy being complicated by preeclampsia is significant, with rates around 13.16% [10]. With the rise in assisted reproductive technology, the incidence of twin pregnancies is increasing, leading to a higher incidence of preeclampsia compared to singleton pregnancies [11,12]. Ding Y et al., noted that in cases of Twin to Twin Syndrome (TTTS), there was no significant difference in Resistance Index (RI) and Systolic/Diastolic (S/D) values between twins on ultrasonography, but neonatal outcomes were poorer, with increased death rates and lower birth weight, length, and Appearance, Pulse, Grimace, Activity, and Respiration (APGAR) scores. In cases of selective Intrauterine Growth Restriction (sIUGR), the larger twin had higher Pulsatility Index (PI) and Resistance Index (RI) but lower S/D ratios [13].
In another case, a 37-year-old with IVF-conceived DCDA twins and a history of previous LSCS presented a complex clinical scenario involving significant obstetric haemorrhage, necessitating an emergency subtotal obstetric hysterectomy. The management of severe PPH in present patient required a multidisciplinary approach, including intensive care support and judicious use of blood products. The present case underscores the importance of preparedness for surgical interventions in managing severe obstetric emergencies and highlights the potential complications associated with IVF pregnancies and previous caesarean sections. Research indicates a high rate of twin pregnancy among IVF patients, which can complicate the management of PPH [14]. Combs CA et al., found factors such as preeclampsia, nulliparity, twins, induction, prolonged labour, and augmentation to be independent risk factors for uterine atony, a common cause of PPH [15].
The management of retained placenta in a 35-year-old primigravida at term illustrates the challenges associated with this obstetric complication. The stepwise approach, which includes the administration of uterotonic agents and manual removal of the placenta under anaesthesia, demonstrates the necessity for a methodical and patient-centered approach. The present case highlights the importance of individualised care plans and close monitoring to manage retained placenta effectively. The incidence of retained placenta varies globally, affecting about 0.1% of deliveries in less developed countries but up to 3% in more developed regions, where it is rarely associated with mortality [16]. Butureanu T et al., described, during a transvaginal ultrasound examination in a case of retained placenta and arteriovenous malformation, a uterus with an inhomogeneous structure. A heterogeneous area was observed with a colour Doppler signal throughout the entire thickness of the anterior wall, extending into the endometrial cavity [17].
The case of uterine rupture in a 32-year-old multigravida at term underscores the critical need for rapid diagnosis and intervention. Despite a history of previous normal vaginal deliveries, the risk of uterine rupture remained, emphasising that this catastrophic event can occur even without classical risk factors such as previous cesarean sections. Prompt surgical management was crucial in addressing the rupture and preventing further complications. Uterine rupture, although rare, occurs in approximately 1 in 5,000 to 7,000 births and is increasing worldwide [18-20]. Quick surgical intervention and resuscitation are vital for survival, with maternal mortality rates higher for ruptures of unscarred uteri compared to scarred uteri [21,22].
The successful medical management of a scar ectopic pregnancy using methotrexate in a 27-year-old female with a history of previous cesarean sections showcases a less invasive alternative to surgical intervention. Consistent monitoring of β-hCG levels and a favorable response to medical treatment highlight the potential for non-surgical management in appropriately selected cases. Early detection is critical for successful outcomes. The incidence of cesarean scar pregnancies is low but increasing, with reported rates of 0.05-0.06% among normal pregnancies and 0.15% among those with previous cesarean sections [23,24]. The success rate of local methotrexate injection in managing such cases can be high, particularly with early diagnosis, as evidenced by success rates ranging from 54-94% in various studies [25-27].
Conclusion(s)
The present series of case reports highlights the critical challenges and complexities in managing severe obstetric emergencies. The cases emphasise the importance of timely intervention, multidisciplinary care, and comprehensive management strategies. From severe preeclampsia and emergency hysterectomy in multifoetal pregnancies to retained placenta, uterine rupture, and scar ectopic pregnancy, each scenario underscores the necessity of rapid diagnosis, aggressive treatment, and coordinated care. The successful outcomes in these high-risk situations illustrate the pivotal roles of specialised medical teams, vigilant monitoring, and effective clinical decision-making in reducing morbidity and mortality among pregnant women facing severe complications.
[1]. Vanawalla NY, Ghamande S, Ingle KM, A five-year analysis of eclampsiaJ Obstet Gynecol India 1989 39:513-15. [Google Scholar]
[2]. Suman G, Somegowda S, Maternal and perinatal outcome in Eclampsia in a District HospitalJ Obstet Gynecol India 2007 57:324 [Google Scholar]
[3]. Sing K, Medhi R, Bhattacharjee AK, Book of Abstract, 53rd AICOG, Guwahati 2010 :17 [Google Scholar]
[4]. Chandriole N, Singh S, Dhillon BS, Eclampsia, care hood and management practices at tertiary hospital in India (ICMR), New DelhiBook of Abstract AICOG, Guwahati 2010 :33 [Google Scholar]
[5]. Pal A, Bhattacharjee R, Banerjee Ch, Maternal mortality over a decade in a referral Medical College Hospital, West BengalIndian J Perinatol Reprod Biol 2001 4:10-13. [Google Scholar]
[6]. Verma A, Sharma G, Kashyap M, A retrospective analysis of emergency obstetric hysterectomy: A life-saving interventionCureus 2023 15(10):e4675810.7759/cureus.4675837946885PMC10632065 [Google Scholar] [CrossRef] [PubMed]
[7]. Weeks AD, The retained placentaAfr Health Sci 2001 1(1):36-41.12789132PMC2704447 [Google Scholar] [PubMed]
[8]. Alemu A, Yadeta E, Deressa A, Debella A, Birhanu A, Heluf H, Survival status and predictors of mortality among women with uterine rupture at public hospitals of eastern Ethiopia. Semi-Parametric Survival AnalysisInt J Womens Health 2023 15:443-53.10.2147/IJWH.S40288537006639PMC10054622 [Google Scholar] [CrossRef] [PubMed]
[9]. ACOG Committee on Practice Bulletins Tubal ectopic pregnancy: ACOG practice bulletinClin Manage Guidelines Obstet Gynecol 2018 131(3):e91-103.10.1097/AOG.0000000000002560 [Google Scholar] [CrossRef]
[10]. Han Q, Zheng S, Chen R, Zhang H, Yan J, A new model for predicting the risk of preeclampsia in twin pregnancyFrontiers in Physiology 2022 13:85014910.3389/fphys.2022.85014935464090PMC9024216 [Google Scholar] [CrossRef] [PubMed]
[11]. Baha MSM, JohnHauth M, SteveCaritis M, Marshall D, Lindheimer M, CoraMacPherson P, Hypertensive disorders in twin versus singleton gestationsAm J Obstet Gynecol 2000 182:938-42.10.1016/s0002-9378(00)70350-410764477 [Google Scholar] [CrossRef] [PubMed]
[12]. Wang Y, Wu N, Shen H, A review of research progress of pregnancy with twins with preeclampsiaRisk Manag Healthc Policy 2021 1999-2010.SS.10.2147/RMHP.S30404034040463PMC8140947 [Google Scholar] [CrossRef] [PubMed]
[13]. Ding Y, Li J, Liu W, Anniwaer A, Li B, Chen Y, Ultrasonographic features and pregnancy outcomes of complications in monochorionic twin pregnancy during various pregnancy periodsAltern Ther Health Med 2024 :AT9780 [Google Scholar]
[14]. Wang Y, Shi H, Chen L, Zheng D, Long X, Zhang Y, Absolute risk of adverse obstetric outcomes among twin pregnancies after in vitro fertilization by maternal ageJAMA Netw Open 2021 4(9):e212363410.1001/jamanetworkopen.2021.2363434505887PMC8433605 [Google Scholar] [CrossRef] [PubMed]
[15]. Combs CA, Murphy EL, Laros RK, Factors associated with postpartum haemorrhage with vaginal birthObstet Gynecol 1991 77(1):69-76. [Google Scholar]
[16]. Weeks AD, The retained placentaBest Pract Res Clin Obstet Gynaecol 2008 22(6):1103-17.Epub 2008 Sep 1410.1016/j.bpobgyn.2008.07.00518793876 [Google Scholar] [CrossRef] [PubMed]
[17]. Butureanu T, Balan RA, Socolov R, Ioanid N, Socolov D, Gafitanu D, Retained placenta percreta with acquired uterine arteriovenous malformation—case report and short review of the literatureDiagnostics 2022 12(4):90410.3390/diagnostics1204090435453952PMC9029973 [Google Scholar] [CrossRef] [PubMed]
[18]. Porreco RP, Clark SL, Belfort MA, Dildy GA, Meyers JA, The changing spectre of uterine ruptureAm J Obstet Gynecol 2009 200(3):269.e1-4.10.1016/j.ajog.2008.09.87419136093 [Google Scholar] [CrossRef] [PubMed]
[19]. Ozdemir I, Yucel N, Yucel O, Rupture of the pregnant uterus: A 9-year reviewArch Gynecol Obstet 2005 272(3):229-31.10.1007/s00404-005-0733-315843950 [Google Scholar] [CrossRef] [PubMed]
[20]. Al-Zirqi I, Stray-Pedersen B, Forsén L, Daltveit AK, Vangen S, Uterine rupture: Trends over 40 yearsBJOG 2016 123(5):780-87.10.1111/1471-0528.1339425846698 [Google Scholar] [CrossRef] [PubMed]
[21]. Kapoor DS, Sharma SD, Alfirevic Z, Management of an unscarred ruptured uterusJ Perinat Med 2003 31(4):337-39.10.1515/JPM.2003.04812951891 [Google Scholar] [CrossRef] [PubMed]
[22]. Chauhan SP, Martin JN, Henrichs CE, Morrison JC, Magann EF, Maternal and perinatal complications with uterine rupture in 142,075 patients who attempted vaginal birth after cesarean delivery: A review of the literatureAm J Obstet Gynecol 2003 189(2):408-17.10.1067/S0002-9378(03)00675-614520209 [Google Scholar] [CrossRef] [PubMed]
[23]. Rotas MA, Haberman S, Levgur M, Cesarean scar ectopic pregnancies: Aetiology, diagnosis, and managementObstet Gynecol 2006 107(6):1373-81.10.1097/01.AOG.0000218690.24494.ce16738166 [Google Scholar] [CrossRef] [PubMed]
[24]. Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL, Cesarean scar pregnancy: Issues in managementUltrasound Obstet Gynecol 2004 23(3):247-53.10.1002/uog.97415027012 [Google Scholar] [CrossRef] [PubMed]
[25]. Glenn TL, Bembry J, Findley AD, Yaklic JL, Bhagavath B, Gagneux P, Cesarean scar ectopic pregnancy: Current management strategiesObstet Gynecol Surv 2018 73(5):293-302.10.1097/OGX.000000000000056129850919 [Google Scholar] [CrossRef] [PubMed]
[26]. Hafner T, Aslam N, Ross JA, Zosmer N, Jurkovic D, The effectiveness of non-surgical management of early interstitial pregnancy: A report of ten cases and review of the literatureUltrasound Obstet Gynecol 1999 13(2):131-36.10.1046/j.1469-0705.1999.13020131.x10079493 [Google Scholar] [CrossRef] [PubMed]
[27]. Wang M, Yang Z, Li Y, Chen B, Wang J, Ma X, Conservative management of cesarean scar pregnancies: A prospective randomized controlled trial at a single centreInt J Clin Exp Med 2015 8(10):18972-80. [Google Scholar]