Dermatophytosis, a common fungal infection affecting 20-25% of the world population [1], is prevalent in tropical and subtropical regions, encompasses various clinical forms, with tinea cruris and corporis being the most common and prevalent presentation [1-3]. The prevalence of tinea in India is 27.6%, with tinea cruris and corporis accounting for 78% and 10% of cases, respectively [4]. However, tinea faciei has emerged as a noteworthy concern in dermatological practice. Tinea faciei is a superficial dermatophyte infection affecting the glabrous skin of the face, thus excluding the areas where moustache and beard grow in adult males [5]. In paediatric patients and females, the infection can appear anywhere on the face, including upper lips and chin. This condition poses diagnostic challenges due to its diverse clinical presentations.
The prevalence of tinea in India is currently 27.6%, with tinea faciei accounting for 1.8% of cases, indicating its significance within the dermatological landscape [4]. Moreover, the evolving clinical spectrum of tinea faciei, including atypical presentations that mimic other dermatoses, necessitates a deeper understanding of its epidemiology and associated risk factors. On review of the literature, most of the studies [1-4,6] have included all superficial dermatophytic infections, with limited studies focusing only on tinea faciei [7-11].
The present study aimed to understand and highlight various morphological features (including the atypical presentations), sites of involvement, risk factors, and prevalence of steroid abuse among tinea faciei patients. This will enhance our understanding regarding the nature of the disease and our ability to treat patients better.
Materials and Methods
The present cross-sectional study was conducted in the Department of Dermatology, Venereology and Leprosy, Hind Institute of Medical Sciences, Barabanki, Uttar Pradesh, India, from October 2022 to October 2023. Informed consent was obtained from the study participants.
Sample size calculation: The sample size was calculated using the formula:
n=Zα2p (1-p)/E2
p=prevalence or incidence
Zα=Critical value of Z-score at corresponding α level of significance
(at, α=5%, Zα=1.96)
E=Margin of error=3%=0.03
p=1.8%=0.018 (prevalence of Tinea faciei [4])
n=(1.96)2×0.018 (1-0.018)/(0.03)2
n=75.44≈75
Inclusion criteria: All consecutive patients with the clinical diagnosis of tinea faciei, irrespective of their age, who gave consent to participate, were recruited in the study and questioned regarding their disease.
Exclusion criteria: Patients who did not give consent and those with KOH-negative patients were excluded from the study.
A total of 112 patients were enrolled, of which 75 were included in the study and 37 were excluded. Patients with KOH examination showing positive findings for the presence of dermatophytes were included in the study.
Study Procedure
Information was collected on age, sex, occupation, total duration of the disease, contact with pets and cattle, family history, exposure to systemic and oral steroids, and previous treatment were collected. Patients were inquired regarding the appearance of the first lesion of tinea, (labelled as index lesion) and noted if present on the face or the site other than the face.
Samples were obtained for direct microscopic examination with KOH. Skin scraping from the periphery of the lesions was collected and subjected to direct microscopic examination with 10% KOH. Different morphologies of tinea faciei, site of involvement on the face, severity of itching, and other affected sites were also noted. Itching intensity was measured using the Numerical Rating Scale (NRS) and categorised into no (0 points), mild (1-3 points), moderate (4-6 points), severe (7-8 points) and very severe pruritus (9-10 points).
Statistical Analysis
Coding, data entry, clearing and compiling were done in Microsoft Excel sheets. Appropriate statistical software, including SPSS software version 29.0, was used for statistical analysis.
Results
The epidemiological data, including gender, age distribution, presence of pets, and exposure to cattle/animals, is summarised in [Table/Fig-1]. The present study comprised 45 males and 30 females, with a mean age of 30.7 years (range: 8 months to 60 years) and a Standard Deviation (SD) of 13.92. There were three children below 10 years of age, with peak incidence observed in the age group of 16-20 years, and only two elderly patients aged 60 years.
Epidemiological profile of patients.
| Variables | n (%) |
|---|
| Gender |
| Male | 45 (60) |
| Female | 30 (40) |
| Age (years) |
| <10 | 03 (4) |
| 10-19 | 16 (21.3) |
| 20-39 | 34 (45.3) |
| 40-59 | 20 (26.6) |
| >60 | 02 (2.6) |
| Presence of pets |
| Yes | 27 (36) |
| No | 48 (64) |
| Exposure to cattle/animals |
| Yes | 35 (46.6) |
| No | 40 (53.3) |
| Family history |
| Present | 21 (28) |
| Absent | 54 (72) |
The site of involvement on the face, various morphological presentations, and severity of itch is summarised in [Table/Fig-2]. Sites other than the face, location of index lesion, presence of steroid abuse, methods of steroid abuse (injection, oral and topical), and description of topical steroid abuse are also mentioned.
Clinical manifestations of patients.
| Parameters | n (%) |
|---|
| Site |
| Cheeks | 45 (60) |
| Forehead | 23 (30.6) |
| Around mouth | 22 (29.3) |
| Nose | 26 (34.6) |
| Around eyes | 26 (34.6) |
| External ear | 22 (29.3) |
| Morphology |
| Central clearing | 11 (14.6) |
| Ill-defined margin | 30 (40) |
| Incomplete circle | 05 (6.66) |
| No scaling | 17 (22.6) |
| Eczematous | 04 (5.33) |
| Psoriasiform | 05 (6.66) |
| Pseudoimbricata | 01 (1.33) |
| Pustular margin | 03 (4) |
| Pustular lesions | 08 (10.66) |
| Pityriasis rosea like | 04 (5.33) |
| Erosive | 05 (6.66) |
| Others like hypo/hyperpigmentation | 35 (46.6) |
| Severity of itching |
| No itching | 02 (2.6) |
| Mild | 21 (28) |
| Moderate | 17 (22.6) |
| Severe and very severe | 35 (46.66) |
| Only face involved | 10 (13.3) |
| Other sites involved along with face | 65 (86.6) |
| Groin | 27 (36) |
| Buttocks | 20 (26.6) |
| Others (including abdomen, chest, inframammary, thigh, waist, legs, scalp, shoulder, foot) | 18 (24) |
| Index lesions |
| Face | 34 (45.33) |
| Groin | 14 (18.66) |
| Buttocks | 11 (14.66) |
| Abdomen | 4 (5.33) |
| Inframammary | 2 (2.66) |
| Thigh | 2 (2.66) |
| Back | 2 (2.66) |
| Others (arm, chest, foot, neck, scalp, shoulder) | 6 (8) |
| Steroid abuse |
| Present | 73 (97.3) |
| Absent | 02 (2.6) |
| Description of topical steroids abuse |
| Only steroids | 10 (13.3) |
| Steroids + antibacterial | 01 (1.33) |
| Steroid + antifungal | 05 (6.66) |
| Steroid + antifungal + antibacterial | 48 (64) |
| Method of steroid use |
| Topical steroids | 64 (85.3) |
| Oral steroids | 25 (33.3) |
| Injectable steroids | 02 (2.6) |
The risk factors associated with tinea faciei seen in the present study are other site involvement along with face: groin, 27 (36%), buttocks, 20 (26.6%), and others, 18 (24%) including abdomen (2), chest, inframammary area, thigh, waist, legs, scalp, shoulder, and foot. The most common occupation was housewives, 18 (24%) cases, followed by students, 15 (20%) cases, unemployed, 12 (16%) cases, farmers, 8 (10.6%) cases, labourers, 7 (9.3%) cases, shopkeepers, 5 (6.6%) cases, cooks, 4 (5.3%) cases and others, 6 (8%) cases. A positive family history was noted in 21 (28%) cases, exposure to pets in 27 (36%) cases, and exposure to cattle in 35 (46.6%) cases. Steroid abuse in the form of topical, oral, and injectable steroids was present in 73 (97.3%) cases. Co-morbidities, including diabetes and hypertension, were found in only eight patients.
Cheeks were the most commonly involved anatomical site on the face, and the most common morphological features noted in the present study were ill-defined margins, absence of scaling and central clearing [Table/Fig-3,4,5,6,7 and 8].
Tinea faciei- a superficial dermatophyte infection affecting the glabrous skin of the face.
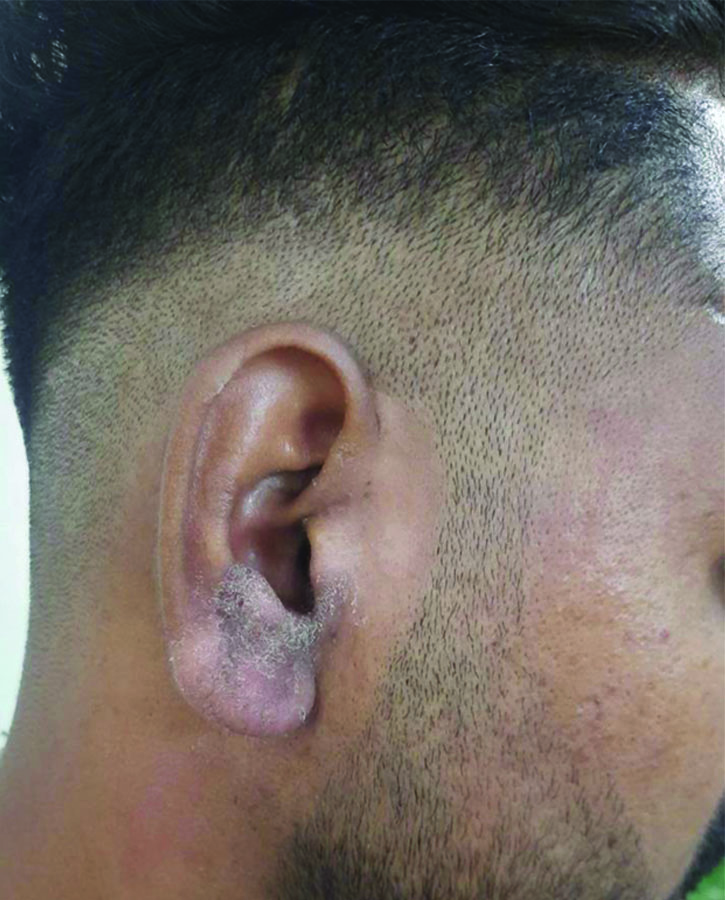
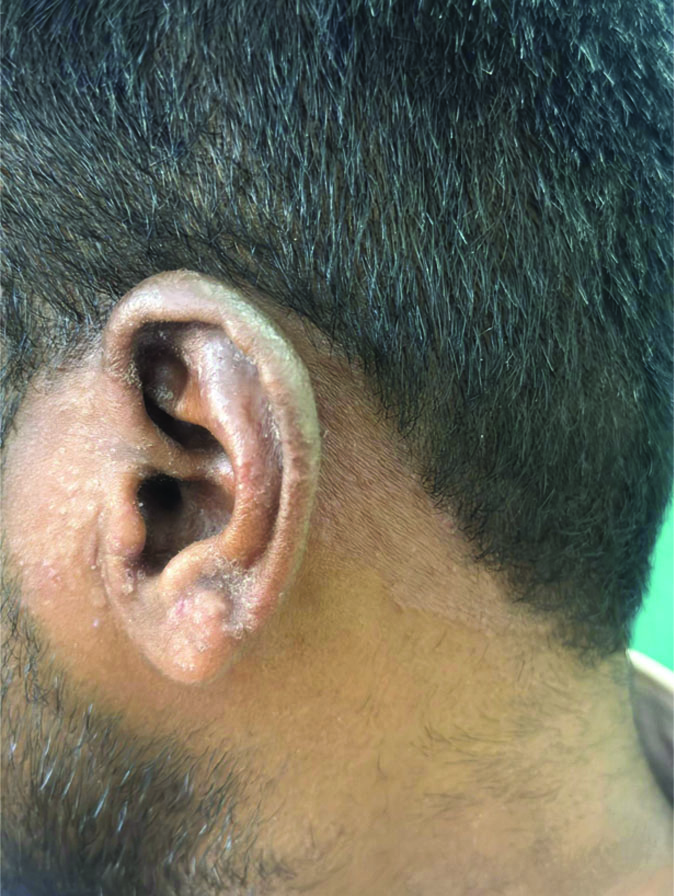
Tinea auricularis including the sole involvement of the ear with no sign of dermatophytosis on the face or elsewhere on the body.
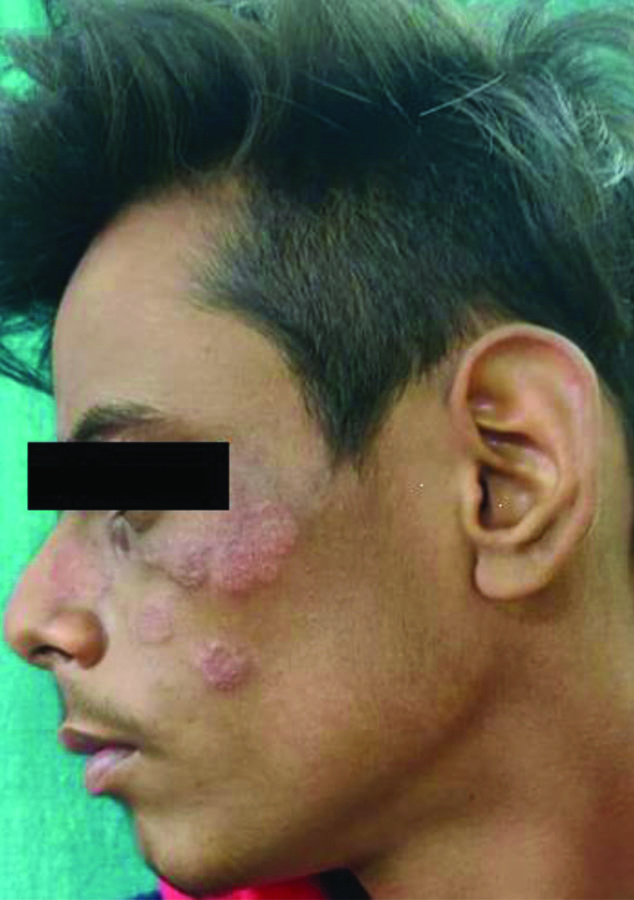
Periocular involvement of tinea lesions presenting only with the involvement of lower eyelids and completely sparing the upper one.
Tinea labialis, in which the facial lesions extend onto the upper lip.
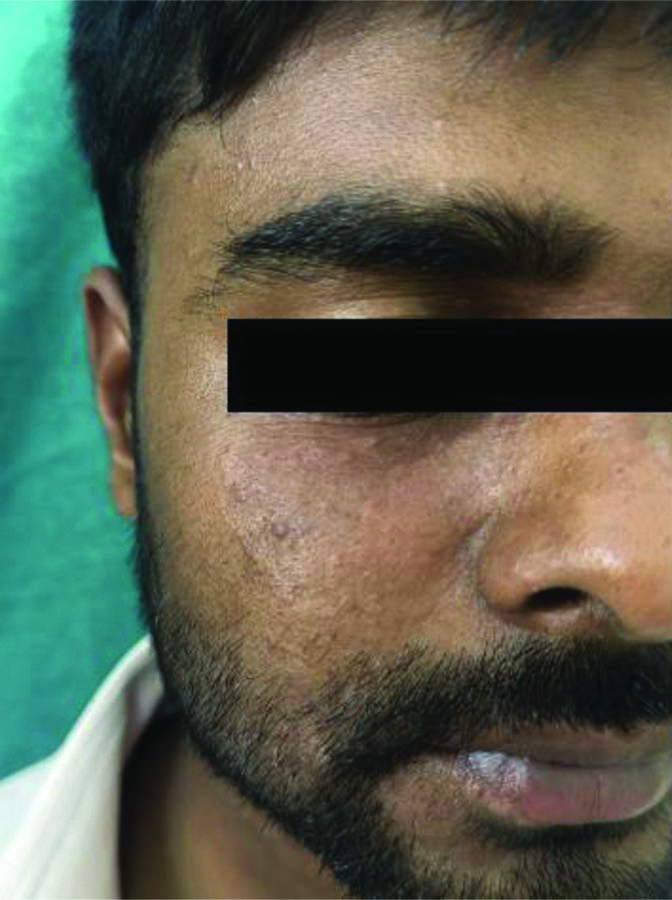
Lesions on the face and neck which also extended to the scalp.
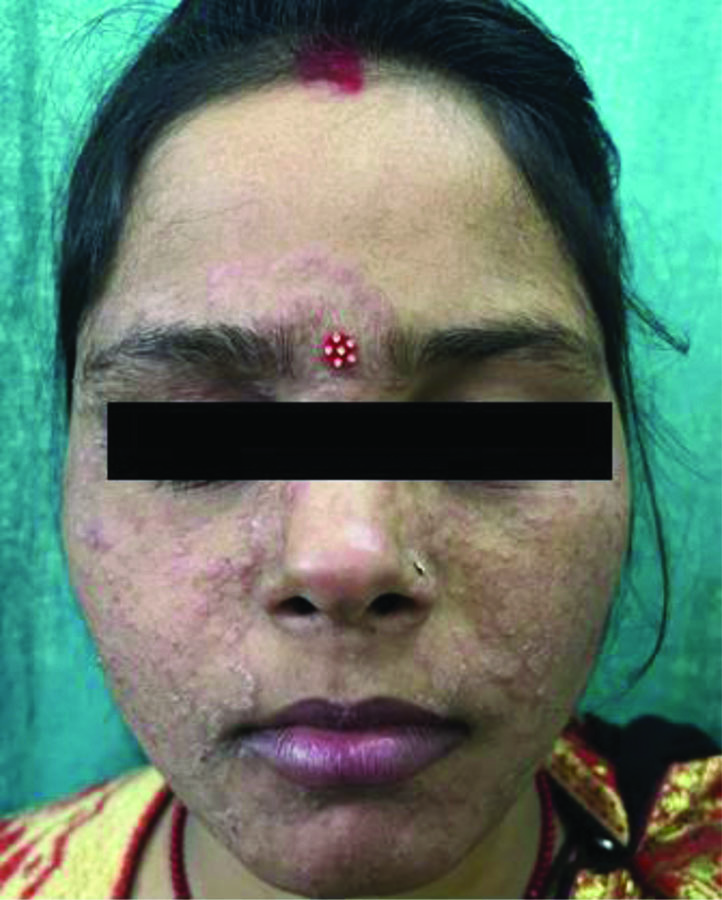
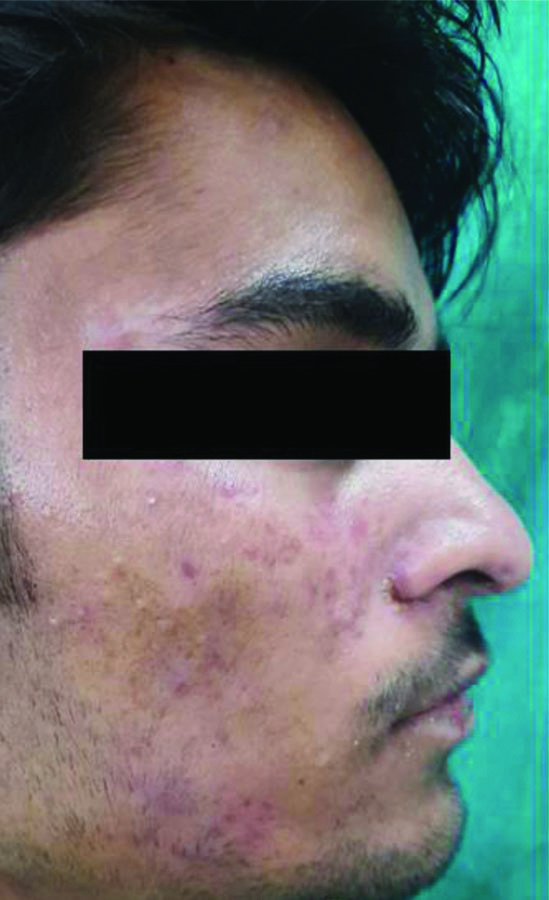
Tinea incognita- the presence of ill-defined margins, absence of scaling, and lack of central clearing, attributed to the inappropriate use of topical steroids.
Discussion
Tinea faciei, historically viewed as a less common type of dermatophytosis comparison to tinea corporis and cruris, has been receiving more focus lately because of its developing range of clinical spectrums and difficulties in diagnosis. The precise prevalence of tinea faciei cannot be determined due to the absence of community-based surveys, as most studies are conducted in hospitals [6]. In addition, a few studies have indicated an increasing incidence of tinea faciei in both adults and children [4].
Several efforts have been made to further categorise tinea faciei based on the involvement of specific facial structures. Tinea auricularis includes the sole involvement of the ear with no signs of dermatophytosis on the face or elsewhere on the body [12]. In the present study, there was a single patient who fit this definition. Periocular involvement of tinea lesions, involving either the upper or lower eyelids, was not very uncommon. Approximately 34.6% of the subjects had periocular involvement, with most showed involvement of both the upper and lower eyelids, including canthi. One patient presented only with the involvement of lower eyelids, completely sparing the upper one. Tinea labialis, with lip involvement, is a rare entity [12]. In the present study, although 22 patients had lesions around the mouth area, only a single patient had an extension of the tinea lesion onto the upper lip. One patient presented with lesions on the face and neck that also extended to the scalp.
In the present study, there were 45 males and 30 females. Other studies have found female predominance. Nicola A et al., studied 107 cases of tinea faciei over 20 years, which included 72 females and 35 males [7]. Another study, with a case series of 30 children with tinea faciei, had eight males and 22 females [8].
The age of the patients enrolled ranged from as young as eight months to as old as 60 years. The mean±SD age of the study participants was 30±13.92 years. The peak incidence of tinea faciei was observed in the age group of 16-20 years, emphasising the vulnerability of adolescents and young adults to this dermatophytosis, possibly due to factors such as increased social interactions, greater outdoor activities, and climate change leading to more perspiration over the face. The rise in tight-fitting and synthetic fabric clothing among this age group makes them more susceptible to developing dermatophytic infections in covered parts of the body, which can eventually lead to facial involvement. A positive family history is another reason for this because of the epidemic-like situation of dermatophytosis in India. Additionally, the misuse of topical steroids on the face for acne and pigmentation greatly disrupts the skin’s defense mechanism against dermatophytes [6]. However, more research studies are needed in the future to validate these risk factors and investigate the susceptibility and vulnerability of young adults to tinea faciei.
The present findings also shed light on the epidemiological and risk factors associated with tinea faciei. The risk factors associated with tinea faciei seen in the present study are involvement of other sites (such as the groin and buttocks). The most common occupation among patients were housewives, followed by student. Positive family history, exposure to pets and cattle, and steroid abuse (in the form of topical, oral and injectable steroids) were also present. Co-morbidities, including diabetes and hypertension, were found in eight patients. The significant association between other site involvement, pet ownership, occupational exposure to animals, positive family history of tinea infection, tight-fitting clothing, lower socio-economic status and immunosuppressive conditions like diabetes mellitus underscores the role of environmental and genetic factors in disease susceptibility. Singh S et al., conducted a case-control study to assess risk factors for chronic and chronic-relapsing tinea corporis, tinea cruris, and tinea faciei [13]. They found a positive association with pre-existing diabetes mellitus, family history of tinea, and personal history of cooking food.
The most commonly involved anatomical site in the face is the cheeks, followed by the areas around the eyes and nose, the forehead, around the mouth and the external ear. Kushwaha P et al., reported that the most common site on the face in children and females is the cheek, while in males, it is the forehead and temple area [9]. Periocular dermatophytic infection is an extremely rare condition, and this atypicality further increases with isolated involvement [14]. Additionally, the diagnosis of periocular tinea is often challenging due to its lack of conventional morphology in most cases [14].
Ten of these patients had tinea affecting only the face, while the involvement of sites other than the face was seen in the rest of 65 patients. Dermatologists can be perplexed when diagnosing due to the involvement of the cheek and nose or when tinea faciei is the sole affectation, as symptoms can worsen with sun exposure and may mimic other photodermatoses, like rosacea and cutaneous lupus erythematosus. This similarity frequently results in misdiagnosis and a worsening of symptoms from wrongly prescribed topical corticosteroids [10].
Amongst those with sites other than face, the most commonly affected site was the groin, in 27 patients, then the buttocks in 20 patients, followed by other sites like the inframammary area, thighs and abdomen. The initial index lesion involving the face was found in 34 patients. Previous literature has documented cases of dermatophytic infections occurring simultaneously in other body sites alongside tinea faciei, which aligns with what the authors have observed [10]. It’s important to note, however, that the index lesion was reported on the face in approximately fifty percent of our patients, which is an exposed area. This contrasts with the earlier belief that dermatophytes prefer occluded areas of the body and later involve the face following auto-inoculation [11].
The present study evaluated the various classical and atypical clinical morphologies of tinea faciei. The classical morphology of tinea is defined as well-defined, centrifugally spreading annular lesions with central clearing and an advancing scaly border. In the present study, central clearing was seen in only 11 patients. The most common atypical morphology of the lesions showed an ill-defined margin in 30 patients, followed by an absence of scaling in 17 patients, pustular lesions in eight patients, incomplete circle, psoriasiform lesions and erosions, which were seen in five patients each. Pityriasis rosea-like and eczematous lesions were noted in four patients each, while pustular margins were observed in three patients. The severity of itching varied from mild in 21 patients to moderate in 17 patients, and severe to very severe in 35 patients. One notable observations from the present study is the diverse clinical presentations of tinea faciei, ranging from typical erythematous plaques with well-defined borders to atypical morphologies mimicking other dermatological conditions. This diversity underscores the importance of clinical acumen and mycological confirmation in accurately diagnosing tinea faciei, especially in cases with ambiguous clinical features. The presence of ill-defined margins, absence of scaling and lack of central clearing, commonly observed in the present study patient cohort, highlight the phenomenon of tinea incognita, often attributed to the inappropriate use of topical steroids. Tinea faciei has been described to have a higher incidence in tinea incognito compared to other dermatophytoses [15-17]. Dermatophytosis typically follows a prolonged non inflammatory course and mainly affects the superficial keratinous layers of the skin. It seldom penetrates beneath the skin’s surface, leading to atypical clinical manifestations. Although the primary cause is thought to be topical corticosteroids, which reduce local immunity and enable fungi to invade beyond the superficial skin layers, it is important to consider the aggressiveness of the invading pathogen, the systemic immunity of the host, complicated facial anatomy of skin, and vulnerability of face to more sun exposure in contributing to the unusual presentations of tinea faciei [4,10,18-20].
This corresponds to several such propensities of tinea faciei where it mimicked conditions such as discoid lupus erythematosus [21], rosacea [22], impetigo [23], polymorphic light eruptions [24] and Sweet’s syndrome [18].
Tinea faciei is a condition with complex symptoms that necessitates thorough history-taking to identify contact with animals. A physical examination is crucial to check for involvement of other areas, which can provide important clues [20]. Microbiological examination and histopathology are important for detecting deeply hidden infections and to rule out closely resembling facial dermatoses [6,20]. Creating a KOH mount is a necessary, quick and straightforward step for all suspected facial lesions, whether scaly or non scaly. Mycological culture is used to confirm the diagnosis and provide reliable evidence to support claims in cases where the KOH microscopy result is negative [20].
Steroid abuse was found in 73 patients, with most patients giving a history of only topical steroid usage. A few patients also provided a history of oral or injectable steroid usage. Of particular concern is the high prevalence of steroid abuse among the present study patient cohort, with the majority reporting the use of topical corticosteroids, either alone or in combination with antifungal agents. This highlights the widespread misuse of topical steroids in dermatological practice, often driven by patients’ desire for rapid symptomatic relief and cosmetic improvement. The consequences of steroid abuse in tinea faciei, including disease exacerbation, altered clinical morphology and therapeutic resistance, underscore the imperative for judicious prescribing practices and patient education regarding the appropriate use of topical corticosteroids.
Limitation(s)
The fungal culture was not performed, and it was an Outpatient Department-based study, and not a true representation of the population.
Conclusion(s)
In conclusion, the present study offers important insights into various aspects of tinea faciei, including its occurrence, epidemiology, risk factors and the role of steroid abuse. The authors have observed that tinea faciei can show unusual symptoms, and recognising these atypical signs can help clinicians diagnose the condition early. This is particularly useful when the disease does not match the typical symptoms and may be mistaken for other conditions. Moreover, the study highlights that many patients use steroids to get quick relief, which can complicate the diagnosis. Future research with larger groups of people is needed to build on these findings. Such studies could help us gain a deeper understanding of tinea faciei, improve diagnostic techniques and develop better treatment strategies.