Introduction
The T2DM is a chronic metabolic disorder characterised by high blood sugar levels resulting from the body’s inability to properly use insulin. It is a prevalent condition worldwide, and its incidence has been steadily increasing over the past few decades [1]. Hyperglycaemia is a substantial risk factor for myocardial infarction, stroke, microvascular events and death in T2DM, which is a progressive illness [2]. The increasing prevalence of T2DM in recent decades cannot be solely attributed to genetic factors; environmental factors, including diet and lifestyle, are believed to contribute to this trend [3].
These factors have the ability to alter gene expression without modifying the DNA sequence, a phenomenon known as epigenetics. The term epigenetics describes modifications to DNA that result in variations in gene expression without altering the DNA sequence [4]. Epigenetic modifications involve chemical alterations that influence how the body interprets DNA. Extensive research has been directed towards investigating various epigenetic changes, such as DNA methylation, histone modifications and non coding RNAs. Researchers are looking into the function of epigenetics in the aetiology of T2DM in an attempt to find the missing piece of the disease’s pathogenesis. Beyond DNA methylation, histone acetylation and gene silencing by non coding RNAs, other factors like addition/removal of phosphate groups (phosphorylation), ubiquitin-tagged degradation, disulfide bond formation and sumoylation are also considered as factors affecting epigenetics [5].
While genetic variables can have an impact throughout life and are fixed from birth, modifiable factors can operate as environmental exposures that either amplify or neutralise these genetic impacts [6]. When referring to the turning on or off of genes necessary to create the long-lasting alterations linked to the differentiation between several cell types [7].
It is now evident that other processes are also at work in addition to DNA changes, like methylation of cytosine residues. Complex alterations may also affect the chromatin that surrounds the DNA [8]. The epigenetics changes, such as DNA methylation and histone modifications, can impair the body’s ability to regulate blood sugar levels, leading to the characteristic hyperglycaemia seen in T2DM [9]. In addition to DNA modifications, non coding RNA molecules, such as microRNAs and long non coding RNAs, also play a role in the epigenetic regulation of gene expression. These molecules can interact with messenger RNAs (mRNAs) and either enhance or suppress their translation into proteins [10]. Disruptions in the balance between these regulatory RNAs can have profound effects on cellular function and contribute to the development of metabolic disorders like T2DM [11].
Furthermore, environmental factors, such as diet and exercise, can influence the epigenetic landscape and contribute to the development of T2DM. For example, a previous study found that a high-fat diet can induce changes in DNA methylation patterns in adipose tissue, muscle and liver, which can affect the expression of genes involved in energy metabolism [12]. Similarly, regular physical activity has been associated with alterations in DNA methylation and histone modifications, leading to improved insulin sensitivity and glucose utilisation [13].
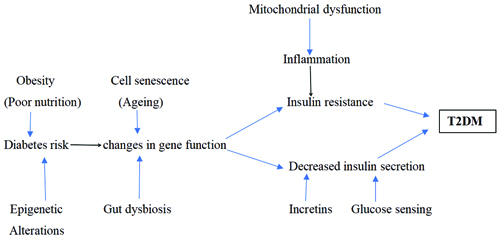
Studies on animals have shown that the expression of genes in chromatin that has been altered by histone Post-translational Modifications (PTM) contributes to the development of diabetes sequelae [14]. Overall, understanding the role of epigenetics in the development of T2DM is critical for the development of effective prevention and treatment strategies. By elucidating the molecular mechanisms underlying this complex metabolic disorder, researchers can identify potential therapeutic targets and biomarkers for early diagnosis. Various factors involved in causing the pathogenesis of T2DM are exhibited in [Table/Fig-1] [15].
Overview of various factors leading to the pathogenesis of T2DM [15].
The present study aimed to explore the role of epigenetics and its mechanisms in the aetiology of T2DM. A thorough search of the literature was performed to find epigenetic alterations linked to T2DM. To provide light on our present understanding of the molecular pathways implicated in the pathogenesis of T2DM, pertinent literature was evaluated, reviewed and reported.
Epigenetics in Human Disease
Through translational applications and the intricate interplay between genes and the environment, epigenetics provides crucial insights into the genesis and course of many diseases, like T2DM, cancer, cardiovascular disorders, neurological conditions (such as Alzheimer’s disease) and autoimmune disorders (like rheumatoid arthritis), even beyond its molecular complexity [16]. Biochemical processes ranging from ageing and disease susceptibility to embryonic development and tissue differentiation are remarkably controlled by epigenetic pathways. Stable epigenetic inheritance and regulatory plasticity dynamically interact in a variety of contexts, including the genesis of illnesses, responses to physiological cues and cellular reprogramming events [17]. Environmental factors, such as stress, diet and chemical exposure, can have a substantial impact on the risk and course of disease. These factors can also produce major changes in epigenetic profiles [18].
Although genetics have a significant impact on the pathogenesis of T2DM, it is pivotal to understand the factors (chromatin level) that regulate blood glucose, starting with the site of insulin production and then to various epigenetic modifications. Hereby, the authors have discussed the role of beta cells of the pancreas in regulating T2DM, along with the vast genetic profiles and epigenetic alterations, like DNA methylation, histone modifications and non coding RNAs, involved in the development and succession of T2DM.
The role of beta cells of the pancreas in T2DM: Alpha and beta cells, two different types of pancreatic islet cells, can malfunction and cause dysregulated glucose homeostasis, as seen in T2DM. Insulin resistance and beta cell dysfunction are made worse by persistent low-grade inflammation, which is characterised by elevated levels of proinflammatory cytokines such Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-1β (IL-1β) [19]. Impaired autophagy, a cellular process that releases damaged proteins and organelles, aggravates the pathogenesis of T2DM [20]. Additionally, misfolded proteins cause oxidative damage and beta cell accumulation. The deregulation of microRNAs (miRNAs) in beta cells further exacerbates impaired insulin production and beta cell death in T2DM. Insulin sensitivity and beta cell function are significantly impacted by environmental factors, including diet, sedentary lifestyle and pollution exposure, in individuals with T2DM [21].
Research on mice that compared the importance of insulin and Insulin-like Growth Factor 1 (IGF-1) in high-fat diets found that the mice developed insulin resistance [22]. Furthermore, abnormalities in insulin signalling play a functional role in pancreatic beta cells by aiding in the aetiology of T2DM [23]. Hyperinsulinemia, a condition brought on by T2DM, is directly linked to an increased risk factor for Pancreatic Intraepithelial Neoplasia (PanIN), a type of cancer [24]. In streptozotocin-induced hyperglycaemia, research on mice shows that higher level of plasma IL-18 increase beta-cell size and proliferation, which in turn increases insulin output [25]. Molecular mechanisms have demonstrated that, in the context of diet-induced hyperinsulinemia and obesity, the expression of insulin receptors in KRAS G12D is required for hyperinsulinemia-driven PanIN formation [26]. This was also linked to increased translation of digestive enzyme proteins, induction of local inflammation, and in-vivo PanIN metaplasia [27]. The combined effects of oxidative stress, Endoplasmic Reticulum (ER) stress, and decreased autophagy implicates a direct correlation between reduced beta cell mass and pathogenesis of T2DM [28]. A recent study compared T2DM patients and healthy controls for Body Mass Index (BMI) in the Japanese population showed that beta cell apoptosis further leads to reduced beta cell mass [29]. A study conducted on European population discusses that there is relatively lesser beta cell mass in T2DM compared with normal population [30].
Alterations in genes involved in the pathogenesis of T2DM: Several genes have been connected to the onset of T2DM [31]; variations in the expression levels or sequencing of these genes can affect the likelihood and progression of the disease [Table/Fig-2] [32].
Genes associated with pathogenesis of T2DM and their polymorphism [32].
| Gene | Polymorphism | Risk allele/genotype | Frequency |
|---|
| ABCC8 | exon 22 C/T (codon 761) | T | 0.01-0.03 |
| ABCC8 | intron 24 -3T/C | C | 0.43-0.49 |
| GCGR | Gly40Ser | Ser | 0.01-0.02 |
| GCK | 3’ CA repeat | z+4 allele | 0.12 |
| GCK | 5 CA repeat | 2 allele | 0.04 |
| INS | VNTR | Class III allele= large | 0.33 |
| INSR | SstI | 5.8 kb allele | 0.04-0.06 |
| INSR | Val985Met | Met | 0.01 |
| IPF1 | Asp76Asn | Asn | 0.01 |
| KCNJ11 | Glu23Lys | Lys | 0.37 |
| PPARG | Pro12Ala | Pro | 0.91 |
| FRDA | GAA repeat | 10-36 repeats | 0.03-0.04 |
| GYS1 | XbaI | A2 site present | 0.04 |
The INS gene: This gene is one of the well-studied genes associated with T2DM because it generates insulin, the hormone necessary for glucose uptake and metabolism. Variations in the INS gene have been linked to decreased insulin production and susceptibility to T2DM. A key gene associated with insulin sensitivity and adipocyte growth, Peroxisome Proliferator-activated Receptor Gamma (PPARG), has also been connected to T2DM. The PPARG gene has been implicated in the development of insulin resistance and T2DM. Additionally, genes linked to insulin signalling pathways and pancreatic beta-cell function, such as Glucokinase (GCK), Hepatocyte Nuclear Factor 1 Alpha (HNF1A) and Insulin Receptor Substrate 1 (IRS1), have also been linked to genetic risk factors for T2DM. Variants in these genes may worsen the pathogenesis of T2DM by affecting insulin synthesis, insulin signalling, and glucose sensing [33]. Recent developments in genomic technologies, such as Genome-wide Association Studies (GWAS), have facilitated the identification of novel genetic loci associated with T2DM [32,33]. These studies have improved our knowledge of the underlying biological mechanisms of T2DM by identifying novel genes and genetic variants that raise the risk of the disease.
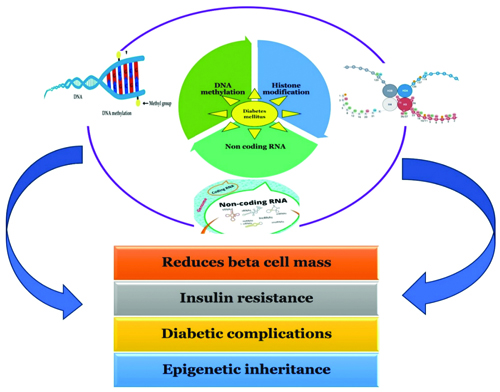
Epigenetic mechanisms in T2DM: T2DM is largely influenced by epigenetic pathways, which offer a comprehensive understanding of the disease that goes beyond hereditary predispositions [7]. In response to various internal and environmental stimuli, these mechanisms which include DNA methylation, histone modifications and non coding RNAs combine to control gene expression patterns, which eventually contributes to the pathophysiology of T2DM, as shown in [Table/Fig-3] [34]. The complex interactions among histone modifications, non coding RNAs and DNA methylation add to the multifactorial character of T2DM, underscoring the significance of epigenetics in clarifying the underlying molecular mechanisms of the illness [Table/Fig-3]. Understanding these epigenetic pathways has significant potential for the development of innovative therapeutic approaches and individualised treatment plans with the goal of reducing the worldwide burden of T2DM has significant potential when these epigenetic pathways are well understood [35].
Diagram illustrating the impact of non coding RNAs, histone changes, and DNA methylation on epigenetic pathways influencing T2DM [34].
DNA methylation in T2DM: DNA methylation, one of the most extensively studied epigenetic modifications, involves the addition of methyl groups to cytosine residues within Cytosine phosphate Guanine (CpG) dinucleotides, primarily situated in gene promoter regions [36]. The alteration in DNA methylation patterns can result in the transcriptional silencing or activation of specific genes relevant to T2DM. For instance, hypermethylation of promoter regions of genes such as PDX1 and INS has been linked to impaired insulin secretion and β-cell dysfunction, key features of T2DM. DNA Methyltransferase 1 (DNMT1) is assumed to be the primary maintenance methyltransferase of the three DNMTs, with enzymatic activity probably by Ubiquitin-like with {Plant Homeodomain (PHD)} and Ring Finger domains 1 (UHRF-1), help because they recognise the hemi-methylated sites [37]. DNMT3a and DNMT3b are involved in the maintenance of DNA methylation patterns, which would mostly methylate the sites that are probably missed by DNMT1. Numerous investigations that examined the connection between alterations in DNA methylation and gene expression in islets from donors with T2DM using a candidate gene method [38].
In a study, 1,649 CpG sites and 853 genes, including Tcf7l2, Fto, Kcnq1, Irs1, Cdkn1a and Pde7b, were shown to have significantly different DNA methylation in human islets from T2DM donors compared to controls, using the genome-wide Infinium 450K array [39]. The study conducted by Davegårdh C et al., examined the alteration patterns involved in the methylation of human pancreatic DNA among T2DM and non T2DM, well explained the epigenetic mechanisms that contribute to the prevalence of diabetes [40].
A previous study has found DNA methylation of genes such as INS, PDX1, PPARGC1A, and GLP1R in human pancreatic islets of donors with T2D and non diabetic controls. These genes may be linked to the development of T2DM [41]. Islets from the T2DM have increased DNA methylation compared to those from normal individuals [42]. Studies found altered DNA methylation in adipose tissue, liver and pancreas islets. Due to T2DM being polygenic, specific CpG sites are unlikely to show methylation [43].
A role for histone modifications: The role of histone modifications, like DNA methylation, acetylation and small non coding RNAs like miRNAs, embarks the phenotypical changes without altering the DNA sequence [44]. PTMs of histones symbolise the epigenome. Genome-wide profiling of histone PTMs has shown that different patterns of particular histone modifications can differentiate critical repeated elements, gene bodies, enhancers and promoters, which are examples of regulatory areas [45].
Role of non coding RNAs and T2DM: Further highlighting the role of epigenetic mechanisms in the disease process, dysregulation of miRNAs and Long non coding RNAs (lncRNAs) has been identified in T2DM Mellitus (T2DM), affecting insulin production, β-cell function, and peripheral insulin sensitivity. lncRNAs are types that have limited protein-coding potential and function as regulatory molecules in epigenetics and at post-transcriptional levels [46]. lncRNAs such as LY86-AS1 and HCG27_201 have been found to be downregulated in individuals with T2DM. Specifically, LY86-AS1 could potentially be used as a biomarker for the pathogenesis of T2DM [47].
Histone deacetylases and T2DM: Histone acetylation and deacetylation are key epigenetic mechanisms. Acetylation favours gene transcription and weakens the interaction between histones and DNA, while deacetylation by Histone Deacetylases (HDACs) inhibits gene transcription. This process is considered a PTM of proteins [48]. One such example of HDACs is the administration of sodium butyrate that ameliorates hyperglycaemia and serum cholesterol levels inT2DM rats induced by high fat and streptozotocin by downregulating the Protein kinase R-like Endoplasmic Reticulum Kinase-C/EBP Homologous Protein (PERK-CHOP) pathway, which is associated with ER stress expressing proteins [49]. Some of the HDAC inhibitors used in inhibition of gene transcription is shown in [Table/Fig-4].
HDAC inhibitors used in histone deacetylation.
| HDAC inhibitor | Animal model | Outcome |
|---|
| 1. Sodium butyrate2. Trichostatin A | Male-C57BL/6J mice | Increased insulin sensitivity and energy expenditure |
| 3. Sodium butyrate | Male calves | Improved insulin sensitivity |
| 4. Tributyrin | C57BL/6 male mice | Attenuated high lipid levels |
| 5. Valproic acid | Streptozotocin induced Sprague Dawley rats | Increased beta cell proliferation |
The Emerging Role of Epigenetics in the Pathophysiology of Gestational Diabetes Mellitus (GDM): A major health risk during pregnancy is Gestational Diabetes Mellitus (GDM), which has impact on the health of both the mother and the foetus. Recent studies have revealed the complex role that epigenetic pathways play in the pathophysiology of GDM, opening up new possibilities for studying and even treating this illness [50,51]. A landmark study titled ’The Emerging Role of Epigenetics in the Pathophysiology of GDM’ explained that DNA methylation, which is pivotal for the maintenance of gene expression in promoter regions, is not restricted only to CpG regions but also includes non CpG regions. Therefore, any epigenetic changes will also influence offspring and its future life [51]. Hence, a complete analysis of epigenetic alterations, due to their reversible nature, must be studied thoroughly to prevent discomfort or poor quality of life in the future. Recent interest has been in downregulating Histone Deacetylase 2 (HDAC2) in GDM patients [52,53]. By eliminating acetyl groups from histone proteins, HDAC2, a member of the histone deacetylase family, is essential in regulating chromatin shape and gene transcription. The expression of genes involved in important metabolic processes is impacted by abnormal histone acetylation patterns caused by deregulation of HDAC2 activity [53].
Further, HDAC2 downregulation affects cellular processes associated with GDM in addition to chromatin remodelling. Interestingly, decreased HDAC2 levels in GDM patients have been linked to impaired mitochondrial activity and increased inflammatory responses [54].
Conclusion(s)
Epigenetics research on T2DM has shown a complicated network of molecular complexity, gene-environment interactions and developmental antecedents. This comprehensive study examined the effects of epigenetic alterations on T2DM from many perspectives, based on fundamental reviews and studies.
In conclusion, the present review offers several insights on T2DM epigenetics. Different perspectives, statistical data and analytical methods help understanding of gene-environment interactions and T2DM development. This knowledge shapes diabetes research and guides the development of targeted therapies, individualised medicine, and well-informed healthcare policy. As we study, epigenetic complexities, we imagine a future where T2DM is understood not only by molecular precision but also by the dynamic interaction between genes, environment and early-life exposures.
[1]. Singh R, Chandel S, Dey D, Ghosh A, Roy S, Ravichandiran V, Epigenetic modification and therapeutic targets of diabetes mellitusBiosci Rep 2020 40(9):BSR2020216010.1042/BSR2020216032815547PMC7494983 [Google Scholar] [CrossRef] [PubMed]
[2]. Fonseca VA, Defining and characterizing the progression of type 2 diabetesDiabetes Care 2009 32(Suppl 2):S1510S15610.2337/dc09-S30119875543PMC2811457 [Google Scholar] [CrossRef] [PubMed]
[3]. Beulens JW, Pinho MG, Abreu TC, den Braver NR, Lam TM, Huss A, Environmental risk factors of type 2 diabetes- an exposome approachDiabetologia 2022 65(2):263-74.10.1007/s00125-021-05618-w34792619 [Google Scholar] [CrossRef] [PubMed]
[4]. Wu Y, Ding Y, Tanaka Y, Zhang W, Risk factors contributing to type 2 diabetes and recent advances in the treatment and preventionInt J Med Sci 2014 11(11):1185-200.10.7150/ijms.1000125249787PMC4166864 [Google Scholar] [CrossRef] [PubMed]
[5]. Jazieh C, Arabi TZ, Asim Z, Sabbah BN, Alsaud AW, Alkattan K, Unraveling the epigenetic fabric of type 2 diabetes mellitus: Pathogenic mechanisms and therapeutic implicationsFront Endocrinol (Lausanne) 2024 15:129596710.3389/fendo.2024.129596738323108PMC10845351 [Google Scholar] [CrossRef] [PubMed]
[6]. Leong A, Porneala B, Dupuis J, Florez JC, Meigs JB, Type 2 diabetes genetic predisposition, obesity, and all-cause mortality risk in the US: A multiethnic analysisDiabetes Care 2016 39(4):539-46.10.2337/dc15-208026884474PMC4806775 [Google Scholar] [CrossRef] [PubMed]
[7]. Mannar V, Boro H, Patel D, Agstam S, Dalvi M, Bundela V, Epigenetics of the pathogenesis and complications of type 2 diabetes mellitustouchREV Endocrinol 2023 19(1):46-53.10.17925/EE.2023.19.1.4637313245PMC10258626 [Google Scholar] [CrossRef] [PubMed]
[8]. Kaplun DS, Kaluzhny DN, Prokhortchouk EB, Zhenilo SV, DNA methylation: Genomewide distribution, regulatory mechanism and therapy targetActa Naturae 2022 14(4):04-19.10.32607/actanaturae.1182236694897PMC9844086 [Google Scholar] [CrossRef] [PubMed]
[9]. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Pathophysiology of type 2 diabetes mellitusInt J Mol Sci 2020 21(17):627510.3390/ijms2117627532872570PMC7503727 [Google Scholar] [CrossRef] [PubMed]
[10]. Fernandes JC, Acuña SM, Aoki JI, Floeter-Winter LM, Muxel SM, Long non-coding RNAs in the regulation of gene expression: Physiology and diseaseNoncoding RNA 2019 5(1):1710.3390/ncrna501001730781588PMC6468922 [Google Scholar] [CrossRef] [PubMed]
[11]. Oo JA, Brandes RP, Leisegang MS, Long non-coding RNAs: Novel regulators of cellular physiology and functionPflugers Arch 2022 474(2):191-204.10.1007/s00424-021-02641-z34791525PMC8766390 [Google Scholar] [CrossRef] [PubMed]
[12]. Keleher MR, Bunting LD, Tresnak TA, Brons G, Fullerton AM, Silverman J, A high-fat diet alters genome-wide DNA methylation and gene expression in SM/J miceBMC Genomics 2018 19(1):88810.1186/s12864-018-5327-030526554PMC6286549 [Google Scholar] [CrossRef] [PubMed]
[13]. Światowy WJ, Drzewiecka H, Kliber M, Sąsiadek M, Karpiński P, Pławski A, Physical activity and DNA methylation in humansInt J Mol Sci 2021 22:1298910.3390/ijms22231298934884790PMC8657566 [Google Scholar] [CrossRef] [PubMed]
[14]. Zhong Q, Kowluru RA, Role of histone acetylation in the development of diabetic retinopathy and the metabolic memory phenomenonJ Biochem 2010 110(6):1306-13.10.1002/jcb.2264420564224PMC2907436 [Google Scholar] [CrossRef] [PubMed]
[15]. Ma Q, Li Y, Wang M, Tang Z, Wang T, Liu C, Progress in metabonomics of type 2 diabetes mellitusMolecules 2018 23:183410.3390/molecules2307183430041493PMC6100487 [Google Scholar] [CrossRef] [PubMed]
[16]. Feinberg AP, The key role of epigenetics in human disease prevention and mitigationN Engl J Med 2018 378(14):1323-34.10.1056/NEJMra140251329617578PMC11567374 [Google Scholar] [CrossRef] [PubMed]
[17]. Cavalli G, Heard E, Advances in epigenetics link genetics to the environment and diseaseNature 2019 571:489-99.10.1038/s41586-019-1411-031341302 [Google Scholar] [CrossRef] [PubMed]
[18]. Skinner MK, Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stabilityEpigenetics 2011 6(7):838-42.10.4161/epi.6.7.1653721637037PMC5703187 [Google Scholar] [CrossRef] [PubMed]
[19]. Cerf ME, Beta cell dysfunction and insulin resistanceFront Endocrinol (Lausanne) 2013 4:3710.3389/fendo.2013.0003723542897PMC3608918 [Google Scholar] [CrossRef] [PubMed]
[20]. Barlow AD, Thomas DC, Autophagy in diabetes: β-cell dysfunction, insulin resistance, and complicationsDNA Cell Biol 2015 34(4):252-60.10.1089/dna.2014.275525665094 [Google Scholar] [CrossRef] [PubMed]
[21]. Mahajan A, Spracklen CN, Zhang W, Ng MCY, Petty LE, Kitajima H, Multi-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translationNat Genet 2022 54:560-72.10.1038/s41588-022-01058-335551307PMC9179018 [Google Scholar] [CrossRef] [PubMed]
[22]. Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krtzfeldt J, Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistanceProc Natl Acad Sci U S A 2007 104(21):8977-82.10.1073/pnas.060870310417416680PMC1885613 [Google Scholar] [CrossRef] [PubMed]
[23]. Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stressWorld J Diabetes 2023 14(3):130-46.10.4239/wjd.v14.i3.13037035220PMC10075035 [Google Scholar] [CrossRef] [PubMed]
[24]. Wood LD, Canto MI, Jaffee EM, Simeone DM, Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatmentGastroenterology 2022 163(2):386-402.e1.10.1053/j.gastro.2022.03.05635398344PMC9516440 [Google Scholar] [CrossRef] [PubMed]
[25]. Zhang X, Luo S, Wang M, Huang Q, Fang W, Li J, IL18 signaling causes islet β cell development and insulin secretion via different receptors on acinar and β cellsDevelopmental Cell 2022 57(12):1496-1511.e6.10.1016/j.devcel.2022.05.01335675813PMC9233156 [Google Scholar] [CrossRef] [PubMed]
[26]. Yan HH, Jung KH, Lee JE, Son MK, Fang Z, Park JH, ANGPTL4 accelerates KRASG12D-Induced acinar to ductal metaplasia and pancreatic carcinogenesisCancer Lett 2021 519:185-98.10.1016/j.canlet.2021.07.03634311032 [Google Scholar] [CrossRef] [PubMed]
[27]. Zhang AM, Xia YH, Lin JH, Chu KH, Wang WC, Ruiter TJ, Hyperinsulinemia acts via acinar insulin receptors to initiate pancreatic cancer by increasing digestive enzyme production and inflammationCell Metab 2023 35(12):2119-35.10.1016/j.cmet.2023.10.00337913768 [Google Scholar] [CrossRef] [PubMed]
[28]. Gallagher EJ, LeRoith D, Hyperinsulinaemia in cancerNat Rev Cancer 2020 20(11):629-44.10.1038/s41568-020-0295-532908223 [Google Scholar] [CrossRef] [PubMed]
[29]. Mizukami H, Takahashi K, Inaba W, Tsuboi K, Osonoi S, Yoshida T, Involvement of oxidative stress–induced DNA damage, endoplasmic reticulum stress, and autophagy deficits in the decline of β-cell mass in Japanese type 2 diabetic patientsDiabetes Care 2014 37(7):1966-74.10.2337/dc13-201824705612 [Google Scholar] [CrossRef] [PubMed]
[30]. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC, Pancreatic beta-cell mass in European subjects with type 2 diabetesDiabetes Obes Metab 2008 10(Suppl 4):32-42.10.1111/j.1463-1326.2008.00969.x18834431 [Google Scholar] [CrossRef] [PubMed]
[31]. Hirschhorn J, Lohmueller K, Byrne E, Hirschhorn K, A comprehensive review of genetic association studiesGenet Med 2002 4(2):45-61.10.1097/00125817-200203000-00002 [Google Scholar] [CrossRef]
[32]. ENCODE Project ConsortiumAn integrated encyclopedia of DNA elements in the human genomeNature 2012 489(7414):57-74.10.1038/nature1124722955616PMC3439153 [Google Scholar] [CrossRef] [PubMed]
[33]. Himanshu D, Ali W, Wamique M, Type 2 diabetes mellitus: Pathogenesis and genetic 571 diagnosisJ Diabetes Metab Disord 2020 19(2):1959-66.10.1007/s40200-020-00641-x33520871PMC7843813 [Google Scholar] [CrossRef] [PubMed]
[34]. Sriraman A, Debnath TK, Xhemalce B, Miller KM, Making it or breaking it: DNA methylation and genome integrityEssays Biochem 2020 64(5):687-703.10.1042/EBC2020000932808652PMC7606623 [Google Scholar] [CrossRef] [PubMed]
[35]. Hossan T, Kundu S, Alam SS, Nagarajan S, Epigenetic modifications associated with the pathogenesis of Type 2 diabetes mellitusEndocr Metab Immune Disord Drug Targets 2019 19(6):775-86.10.2174/187153031966619030114554530827271 [Google Scholar] [CrossRef] [PubMed]
[36]. Bansal A, Pinney SE, DNA methylation and its role in the pathogenesis of diabetesPediatr Diabetes 2017 18(3):167-77.10.1111/pedi.1252128401680PMC5394941 [Google Scholar] [CrossRef] [PubMed]
[37]. Bostick M, Kim JK, Estève PO, Clark A, Pradhan S, Jacobsen SE, UHRF1 plays a role in maintaining DNA methylation in mammalian cellsScience 2007 317(5845):1760-64.10.1126/science.114793917673620 [Google Scholar] [CrossRef] [PubMed]
[38]. Chen T, Ueda Y, Dodge JE, Wang Z, Li E, Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3bMol Cell Biol 2003 23(16):5594-605.10.1128/MCB.23.16.5594-5605.200312897133PMC166327 [Google Scholar] [CrossRef] [PubMed]
[39]. Dayeh T, Volkov P, Salö S, Hall E, Nilsson E, Olsson AH, Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretionPLoS Genet 2014 10(3):e100416010.1371/journal.pgen.100416024603685PMC3945174 [Google Scholar] [CrossRef] [PubMed]
[40]. Davegårdh C, García-Calzón S, Bacos K, Ling C, DNA methylation in the pathogenesis of type 2 diabetes in humansMol Metab 2018 14:12-25.10.1016/j.molmet.2018.01.02229496428PMC6034041 [Google Scholar] [CrossRef] [PubMed]
[41]. Ebrahim N, Shakirova K, Dashinimaev E, PDX1 is the cornerstone of pancreatic β-cell functions and identityFront Mol Biosci 2022 15(9):109175710.3389/fmolb.2022.109175736589234PMC9798421 [Google Scholar] [CrossRef] [PubMed]
[42]. Ling C, Bacos K, Rönn T, Epigenetics of type 2 diabetes mellitus and weight change- a tool for precision medicine?Nat Rev Endocrinol 2022 18(7):433-48.10.1038/s41574-022-00671-w35513492 [Google Scholar] [CrossRef] [PubMed]
[43]. Baca P, Barajas-Olmos F, Mirzaeicheshmeh E, Zerrweck C, Guilbert L, Sánchez EC, DNA methylation and gene expression analysis in adipose tissue to identify new loci associated with T2D development in obesityNutr Diabetes 2022 12(1):5010.1038/s41387-022-00228-w36535927PMC9763387 [Google Scholar] [CrossRef] [PubMed]
[44]. Liu R, Wu J, Guo H, Yao W, Li S, Lu Y, Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targetsMedComm 2020 4(3):e29210.1002/mco2.29237220590PMC10200003 [Google Scholar] [CrossRef] [PubMed]
[45]. Millán-Zambrano G, Burton A, Bannister AJ, Schneider R, Histone post-translational modifications- cause and consequence of genome functionNat Rev Genet 2022 23(9):563-80.10.1038/s41576-022-00468-735338361 [Google Scholar] [CrossRef] [PubMed]
[46]. An T, Fan H, Liu YF, Pan YY, Liu YK, Mo FF, The difference in expression of long noncoding RNAs in rat semen induced by high-fat diet was associated with metabolic pathwaysPeer J 2017 5:e351810.7717/peerj.351828761781PMC5530988 [Google Scholar] [CrossRef] [PubMed]
[47]. Saeidi L, Ghaedi H, Sadatamini M, Vahabpour R, Rahimipour A, Shanaki M, Long non-coding RNA LY86-AS1 and HCG27_201 expression in type 2 diabetes mellitusMol Biol Rep 2018 45(6):2601-08.10.1007/s11033-018-4429-830328000 [Google Scholar] [CrossRef] [PubMed]
[48]. Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent mannerProc Natl Acad Sci U S A 2013 110(7):2647-52.10.1073/pnas.121336411023297220PMC3574934 [Google Scholar] [CrossRef] [PubMed]
[49]. Hu Y, Liu J, Yuan Y, Chen J, Cheng S, Wang H, Sodium butyrate mitigates type 2 diabetes by inhibiting PERK-CHOP pathway of endoplasmic reticulum stressEnviron Toxicol Pharmacol 2018 64:112-21.10.1016/j.etap.2018.09.00230342372 [Google Scholar] [CrossRef] [PubMed]
[50]. Halvatsiotis P, Tsokaki T, Chrelias C, Kassanos D, Domali E, Gazouli M, Methylation profile of genes involved in inflammation, in the blood from pregnancies with maternal preeclampsia due to untreated gestational diabetes mellitusHormones 2019 18:173-78.10.1007/s42000-019-00111-x31154656 [Google Scholar] [CrossRef] [PubMed]
[51]. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH, The Pathophysiology of Gestational Diabetes MellitusInt J Mol Sci 2018 19(11):334210.3390/ijms1911334230373146PMC6274679 [Google Scholar] [CrossRef] [PubMed]
[52]. Qu X, Yu H, Jia B, Yu X, Cui Q, Liu Z, Association of downregulated HDAC 2 with the impaired mitochondrial function and cytokine secretion in the monocytes/macrophages from gestational diabetes mellitus patientsCell Biol Int 2016 40(6):642-51.10.1002/cbin.1059826936353 [Google Scholar] [CrossRef] [PubMed]
[53]. Zhang M, Zhou Y, Zhong J, Wang K, Ding Y, Li L, Current guidelines on the management of gestational diabetes mellitus: A content analysis and appraisalBMC Pregnancy Childbirth 2019 19(1):20010.1186/s12884-019-2343-231196116PMC6567433 [Google Scholar] [CrossRef] [PubMed]
[54]. Boyle KE, Hwang H, Janssen RC, DeVente JM, Barbour LA, Hernandez TL, Gestational diabetes is characterized by reduced mitochondrial protein expression and altered calcium signaling proteins in skeletal musclePLoS One 2014 9(9):e10687210.1371/journal.pone.010687225216282PMC4162568 [Google Scholar] [CrossRef] [PubMed]