Introduction
Less than 20% of women in the reproductive age group suffer from PCOS, a multiorgan endocrine condition [1]. Numerous environmental factors, including exposure to endocrine disruptors (such as Bisphenol A), hereditary factors, malfunctions in pituitary and hypothalamic regulatory mechanisms, metabolic dysfunction and obesity, contribute to the development of PCOS [2]. Several molecular pathways, including Anti-Mullerian Hormone (AMH) production, ovarian and adrenal androgen production, Insulin Resistance (IR) and the quantity and functionality of reproductive hormone receptors, are disrupted by gene polymorphisms known to predispose individuals to PCOS [3].
Numerous metabolic disorders, including obesity, Type 2 Diabetes Mellitus (T2DM), IR, hypertension, dyslipidaemia and Cardiovascular Diseases (CVD), are linked to PCOS. The condition is predisposed to and progresses due to idiopathic pathophysiological processes. PCOS arises from a combination of developmental, environmental, genetic and epigenetic factors [4,5]. Hyperandrogenism is believed to be responsible for many of the characteristics of PCOS. Elevated androgens alter various metabolic pathways in target organs such as Adipose Tissue (AT), liver, skeletal muscle, pancreas and brain [6]. However, the exact mechanisms behind these metabolic alterations remain unclear. Additionally, not all PCOS patients exhibit hyperandrogenism. Some of the questions surrounding this complex disorder may be addressed by microRNAs, which originate from various tissues and change according to the pathogenic stage of the disease.
Small, single-stranded non coding RNAs with a length of 19-25 nucleotides (nt) are known as microRNAs. Urine, peritoneal fluid, bronchial lavage, seminal fluid, tears, colostrum, ovarian Follicular Fluid (FF), plasma, serum, saliva and Cerebrospinal Fluid (CSF) contain microRNAs [7,8]. Exosomes and the microRNA Argonaute 2 protein complex, which prevents them from degrading, are the two ways that microRNAs are released into bodily fluids [8]. According to recent research, microRNAs are considered crucial clinical diagnostic and therapeutic biomarkers for a number of pathological conditions, including cancer (such as ovarian cancer early diagnosis, prognosis and chemotherapy sensitivity), neurodegenerative diseases like Alzheimer’s Disease (AD), CVD, pulmonary disease, PCOS and T2DM [9].
MicroRNAs are expressed in FF, ovarian Theca Cells (TC) and Granulosa Cells (GC) in women with PCOS. Furthermore, white blood cells, urine, synovium and other bodily fluids also express microRNAs. MicroRNAs regulate metabolic processes such as ovulation, steroidogenesis and folliculogenesis [10]. Therefore, microRNAs are significant indicators of PCOS. Additionally, microRNAs have been shown to play a role in regulating biological rhythms, which are crucial for controlling reproduction. Inflammation, oxidative stress and IR have been linked to disruptions in the circadian rhythm [11]. MicroRNAs are a non invasive biomarker that is resistant to ribonuclease hydrolysis and persistent in serum [6,8]. However, there are not many studies on microRNA in the serum of PCOS patients [12].
Investigating the relationship between metabolic changes and microRNA-related phenotypic changes in PCOS is urgently needed. MicroRNAs may serve as useful indicators for tracking the course of the illness and the outcomes of different therapeutic approaches. The purpose of this narrative review is to promote a profile of microRNAs that are consistently and nearly universally altered in almost all PCOS patients. Therefore, microRNAs may pave the way for highly specific and sensitive diagnostic indicators as well as therapeutic targets.
Structure of Ovaries
The ovaries are located on either side of the uterus in the pelvis. They are small, oval-shaped glands that produce oocytes and sex hormones such as progesterone and oestrogen. Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH) regulate the various processes taking place in the ovaries. Each ovary has an inner medulla and an outer cortex. The cortex is thicker and more granular than the medulla because it contains ovarian follicles, each of which houses an oocyte. The medulla is rich in blood and lymphatic vessels, as well as nerve fibers [13].
Structure and Function of Ovarian Follicles
In the ovaries, immature eggs are encased in tiny fluid-filled sacs called ovarian follicles. Each woman has approximately 300,000 to 400,000 follicles at puberty [14]. The antral follicle count is typically between 5 and 10 in each ovary. The diameter of a follicle ranges from 2 to 100 mm [15]. The number of antral follicles decreases as a woman ages. For women between the ages of 25 and 34, the typical antral follicle count is between 9 and 12; for women aged 35 to 40, it is between 5 and 9; and for women aged 41 to 46, it is 5 or fewer [15]. The rate at which follicles shrink increases with age, reducing a woman’s ovarian reserve. Thus, the fertility capacity of a woman is directly impacted when these ovarian follicles are absent or not functioning properly [14,15].
Diagnosis of PCOS
Criteria Followed for Diagnosis
The PCOS is characterised by changes in hormone metabolism, including elevated testosterone production, an increased LH to FSH ratio, elevated adrenal androgen levels, impaired insulin sensitivity, hyperglycaemia and an elevated Body Mass Index (BMI). Elevated AMH levels in PCOS patients play a significant role in the pathophysiology of the disease. Additionally, the ovarian structure is altered in PCOS patients with Polycystic Ovarian Morphology (PCOM), which is characterised by an abnormal number of antral follicles. This may be a result of stimulation by LH on the ovarian follicles, which is linked to their prolonged survival. Moreover, the ovarian stroma and TC exhibit enlargement and hyperplasia in PCOM [16]. Several criteria, including the Rotterdam criteria (2003) [17], the National Institutes of Health (NIH) criteria (1990) [18] and the Androgen Excess (AE)-PCOS Society criteria (2006) [19], are used to diagnose PCOS, with the Rotterdam criteria being the most commonly used [Table/Fig-1].
Diagnostic criteria for PCOS [17-19].
| NIH statement (1990) [18] | ESHRE/ASRMstatement(Rotterdam, 2003) (any two) [17] | AES criteria for diagnosis of PCOS (2006) [19] |
|---|
| Hyperandrogenism and/or hyperandrogenaemia | Oligoovulation or anovulation (amenorrhoea, irregular uterine bleeding) | Hyperandrogenism (hirsutism and/or hyperandrogenaemia) |
| Oligomenorrhoea | Clinical and/or biochemical signs of hyperandrogenism (hirsutism, elevated serum total or free testosterone) | Ovarian dysfunction: oligoovulation or anovulation and/or polycystic ovaries |
| Exclusion of related disorders (including but not limited to 21-hydroxylase-deficiency, thyroid dysfunction, hyperprolactinemia, neoplastic androgen secretion, drug-induced androgen excess, syndromes of severe Insulin Resistance (IR), Cushing syndrome, glucocorticoid resistance) | Polycystic ovaries (by ultrasonography) | Exclusion of other androgen excess or related disorders (including but not limited to 21-hydroxylase-deficiency, thyroid dysfunction, hyperprolactinemia, neoplastic androgen secretion, drug-induced androgen excess, syndromes of severe IR, Cushing syndrome and glucocorticoid resistance) |
ESHRE: European society of human reproduction and embryology; ASRM: American society for reproductive medicine
Women with PCOS express microRNAs that are entirely different from those of healthy women. Therefore, microRNAs might serve as potential biomarkers for PCOS diagnosis and prognosis, as they may be able to distinguish between the different PCOS phenotypes identified by the Rotterdam criteria [20].
microRNAS (miRs)
Biogenesis of microRNA
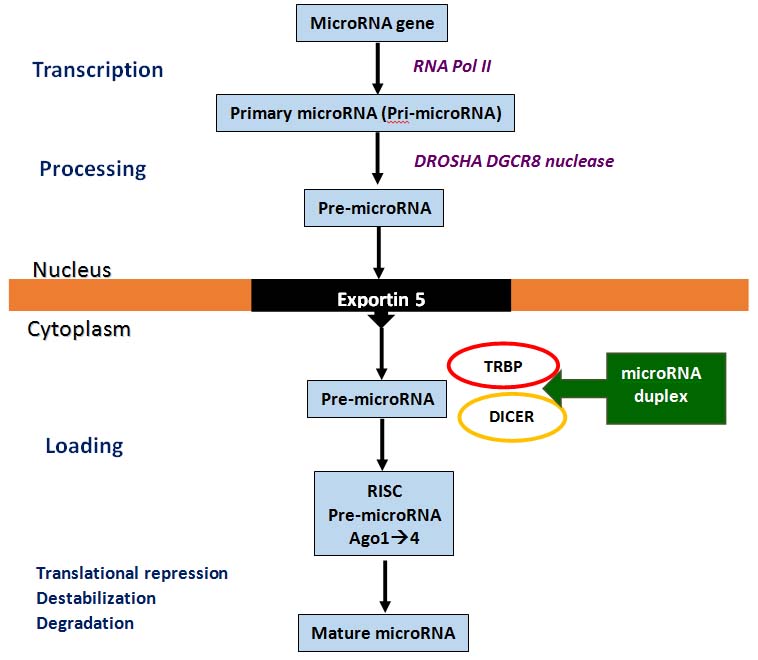
MicroRNA has been identified in both humans and Caenorhabditis elegans. In the nucleus, RNA polymerase II transcribes microRNA genes to create pri-microRNA. A partially processed RNA, called pre-microRNA, is then formed when the DROSHA/DGCR8 nuclease cuts the sequence at the ends. This pre-microRNA is transported from the nucleus into the cytoplasm by Exportin-5. In the cytoplasm, the heterodimeric nuclease (TRBP-DICER) further processes pre-microRNA to produce a 21-22 nucleotide microRNA duplex. After helicase unwinds the selected duplex, one of the two strands is incorporated into the RNA-Induced Silencing Complex (RISC). RISC is composed of four Argonaute proteins (Ago 1-4), with Ago 2 being the only Argonaute protein that cleaves mRNA with slicer activity. The mature functional microRNA is produced through either translational suppression, messenger RNA destabilisation, or degradation [Table/Fig-2] [21].
Biogenesis of microRNA [21].
MicroRNAs Involved in Ovarian Development
The miR-17-92 cluster participates in the development of early ovarian primordial follicles from the original germ cells. It has been discovered that miR-124 plays a role in ovarian development [22]. After the LH surge, miR-21, miR-132 and miR-212 have regulatory roles through the process of transcription [22]. MicroRNA and Transforming Growth Factor-beta 1 (TGF-β1) have been linked to the formation of ovarian follicles [10]. In cultured murine preantral GC, Yao G et al., found that miR-224 is upregulated in response to TGF-β1 [23]. According to Troppmann B et al., miR-513a-3p controls the expression of the LH receptor in GC and acts as a regulator of the LH/Choriogonadotropin Receptor (LHCGR) [24]. Following LH/hCG induction, the expressions of miR-132 and miR-212 are elevated [25]. In GC androgens increase the production of miR-125b, which inhibits apoptotic pathways and enhances GC survival [26]. The early follicular phase exhibits higher levels of serum miR-200b and miR-429 expressions compared to the early luteal phase [27]. MicroRNAs have been revealed to have numerous physiological activities in human beings [Table/Fig-3] [6].
Physiological roles of microRNAs [6].
Sources of microRNA
MicroRNA is present in the whole blood, plasma and serum of PCOS patients. It is isolated from the FF or tissues such as the TC and GC in the ovaries. Additionally, during the pathophysiology of PCOS, microRNAs are produced by the liver, skeletal muscle and AT [28].
Serum/plasma microRNAs in PCOS: Anovulation in women with PCOS is significantly influenced by the altered expression levels of microRNAs. MicroRNAs may play a role in hyperandrogenism, IR and infertility [11]. The development of PCOS is correlated with upregulated miR-27a and miR-301a. While microRNAs are sensitive, miR-27a is particularly specific. The pathogenesis of PCOS is modulated by miR-301a through its regulation of Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ) [29].
Obesity decreases the expressions of miR-21, miR-27b, miR-103 and miR-155 in healthy individuals while increasing them in women with PCOS, according to case-control research that included women with PCOS, healthy women and healthy males. Additionally, there is a positive correlation between these microRNAs and serum free testosterone levels [30]. In PCOS, there is upregulation of miR-126-3p and miR-146a-5p and downregulation of miR-20b-5p, miR-106a-5p and miR-18a-3p. The levels of plasma testosterone are negatively correlated with plasma miR-20b-5p, miR-106a-5p and miR-18a-3p [31].
The LH/FSH ratio and LH levels have an inverse relationship with miR-18a-3p expression. There is a correlation between antral follicle count and AMH concentration with miR-18a-3p, miR-146a-5p and miR-126-3p, but not with oestradiol and FSH levels [31]. The expression of miR-106a-5p, miR-20b-5p and miR-18a-3p is associated with plasma steroid hormones [31]. The expression level of miR-139-5p, which is linked to total testosterone levels, represents the androgenic characteristics of PCOS patients [10]. Serum levels of miR-21, miR-27b and miR-103 are associated with metabolic disorders, PCOS and low-level inflammation [32]. Patients with PCOS who have diabetes have lower levels of miR-130b expression [29]. In women with PCOS, miR-103, miR-27b, miR-155 and miR-21 are associated with T2DM and obesity [28].
Serum levels of miR-4522, miR-324-3p and miR-6767-5p are downregulated in women affected by PCOS. MiR-6767-5p is negatively correlated with fasting glucose. MiR-6767-5p targets the cell cycle and the immune system [33]. IR is associated with lower expression levels of miR-320 in individuals with PCOS [34]. The study by Long W et al., found that miR-222, miR-164a and miR-30c are highly expressed in patients with PCOS. Serum testosterone and miR-164a are negatively correlated, while serum insulin and miR-222 are positively correlated [35]. In PCOS patients with hyperandrogenism, the Homeostatic Model Assessment for IR (HOMA-IR), glucose, insulin and obesity are associated with the levels of miR-1260a, miR-18b-5p, miR-424-5p and let-7b-3p [28].
In PCOS, insulin production and inflammation are regulated by upregulated miR-32, miR-34c, miR-135a, miR-18b and miR-9, as well as by Interleukin 8 (IL-8), synaptogamin 1 and Insulin Receptor Substrate 2 (IRS2) [36]. In PCOS, there is an increase in serum miR-21 and miR-6767-5p and a decrease in serum miR-320. These microRNAs are linked to obesity [22,33,34]. Sathyapalan T et al., claim that although women with PCOS have higher levels of miR-93 and miR-223, these genes are unrelated to insulin, HOMA-IR, HOMA-β, or testosterone [37]. LH levels have an inverse connection with miR-18a-3p, while miR-20b-5p is a marker of IR. These microRNAs target the Mitogen-Activated Protein Kinase (MAPK) signaling pathways, circadian rhythm and axon guidance [31].
In patients with PCOS, let-7i-3pm, miR-5706, miR-4463, miR-3665 and miR-638 are upregulated, while let-7c, miR-124-3p, miR-128 and miR-29a-3p are downregulated. The immune system, ATP binding, MAPK signaling, apoptosis, angiogenesis, response to Reactive Oxygen Species (ROS) and p53 signaling pathways are all impacted by these microRNAs [38]. In comparison to healthy controls, PCOS patients had lower levels of 24 microRNAs, according to Udesen PB et al., [39]. Total testosterone levels and the Waist-Hip Ratio (WHR) were positively correlated with miR-139-5p and miR-376-3p, respectively. The signaling pathways of insulin, interleukin, Gonadotropin-Releasing Hormone (GnRH) receptors and others are targeted by miR-103-3p, miR-139-5p, miR-28-3p and miR-376a-3p [39].
Patients with PCOS exhibit downregulated levels of miR-199b-5p and upregulated expressions of miR-122, miR-193b and miR-194. BMI and miR-193b each make independent contributions to HOMA-IR. These microRNAs target the phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT) pathway, insulin signaling, neurotrophin signaling, glucose metabolism, ovarian follicle development pathways and actin cytoskeleton control [40]. Liu Y et al., claim that miR-151a-5p, miR-4488 and miR-223-3p are unique to PCOS and also suggest a higher likelihood of developing PCOS. The insulin-regulated metabolic pathways involve these microRNAs. While miR-4488 is downregulated in PCOS, miR-151a-5p and miR-223-3p are upregulated. AMH, FSH and LH have positive correlations with miR-151a-5p and miR-4488, whereas FSH, LH and Dihydroepiandrosterone Sulfate (DHEA-S) have negative correlations with miR-223-3p [41]. In women with PCOS, let-7b-3p correlates with AMH [28].
Serum insulin levels are linked to miR-222, while serum testosterone is negatively correlated with miR-146a [31,35]. Higher levels of miR-141 and miR-200c expression may play roles in the aetiology of PCOS. Fertility issues in women with PCOS are associated with low expressions of these microRNAs [42]. miR-146a is elevated in the serum, plasma, FF and GC of patients and it is found to regulate inflammation and the immune response. PCOS and healthy controls exhibit different genotypes and allele frequencies of the miR-146a polymorphism (rs2910164). Because miR-146a can be upregulated, mutations in this gene increase the likelihood of developing PCOS [43]. Polymorphisms in miR-146a rs2910164 and miR-222 rs2858060 may be significant genetic risk factors for the emergence of PCOS in the Iranian population [Table/Fig-4] [10,22,27-31,33-41,43].
Blood microRNAs involved in the various pathogenic processes of PCOS [10,22,27-31,33-41,43].
| MicroRNAs in peripheral blood | Pathogenesis involved | Reference |
|---|
| miR-27a, miR-301a, miR-141, miR-200c | PCOS development | [29] |
| miR-200b, miR-429 | Anovulation | [27] |
| miR-146a-5p, miR-126-3p | Anovulation and polycystic ovaries | [31] |
| miR-301a | Through PPAR-κ modulates PCOS pathogenesis | [29] |
| miR-18a-3p | Associated with LH level and LH/FSH ratio | [31] |
| miR-18a-3p, miR-146a-5p, miR-126-3p | Associated with AFC and AMH levels | [31] |
| miR-106a-5p, miR-20b-5p, miR-18a-3p | Associated with plasma steroid hormone levels | [31] |
| miR-139-5p | Reflects androgen profile in PCOS | [10] |
| miR-21, miR-27b, miR-103 | PCOS, metabolic disorders, inflammation | [31] |
| miR-130b | Diabetic PCOS individuals | [29] |
| miR-103, miR-27b, miR-155, miR-21 | Associated with obesity and Diabetes Mellitus (DM) in PCOS | [30] |
| miR-6767-5p | Target cell cycle. Immune system. Associated with glucose | [33] |
| miR-320, miR-93, miR-222, miR-223 | Associated with Insulin Resistance (IR) | [34,35,37] |
| miR-164a, miR-146a, miR-20b-5p, miR-106a-5p, miR-18a-3p, miR-139-5p | Associated with serum testosterone | [31,35] |
| miR-27a, miR-320, miR-1260a, miR-18b-5p, miR-424-5p, let-7b-3p | Associated with glucose, insulin, obesity, HOMA-IR | [28,30,31,34] |
| miR-32, miR-34c, miR-135a, miR-18b, miR-9 | Regulate insulin production, Interleukin (IL)-8, synaptogamin 1, IRS2 | [36] |
| miR-21, miR-6767-5p | Obesity | [22,33] |
| miR-20b-5p, miR-18a-3p | miR-20b-5p is a marker of IR; Association of miR-18a-3p and LH levels. Target circadian rhythm and MAPK pathway | [31] |
| let-7i-3pm, miR-5706, miR-4463, miR-3665, miR-638 | Role in immune system, ATP binding, MAPK signalling, apoptosis, angiogenesis, response to ROS and p53 signalling pathways | [38] |
| miR-376-3p | Positive association with Waist-Hip Ratio (WHR) | [39] |
| miR-103-3p, miR-139-5p, miR-28-3p, miR-376a-3p | Target signalling of insulin, IL, GNRH receptor | [39] |
| miR-122, miR-193b, miR-194 | Target glucose metabolism, ovarian follicle development, signalling of insulin, neurotrophins, PI3K/protein kinase B AKT | [40] |
| miR-151a-5p, miR-4488, miR-223-3p | Insulin signalling. Increased risk of PCOS | [41] |
| miR-151a-5p, miR-4488 | Associated with AMH, FSH and LH | [41] |
| miR-223-3p | Associated with FSH, LH and DHEA-S | [41] |
| let-7b-3p | Correlates with AMH | [28] |
| miR-222 | Associated with serum insulin levels | [35] |
| miR-146a | Regulates immune response and inflammation and is upregulated in serum, plasma, FF and GC | [31] |
| miR-146a rs2910164 and miR-222 rs2858060 SNP | Genetic risk for PCOS development in Iranians | [43] |
PCOS: Polycystic ovarian syndrome; PPAR: Proliferator-activated receptor gamma; LH: Luteinizing hormone; FSH: Follicle-stimulating hormone; AFC: Antral follicle count; AMH: Anti-mullerian hormone; MAPK: Mitogen-activated protein kinase; FF: Follicular fluid; GC:Granulosa cells; ROS: Reactive oxygen species; GNRH: Gonadotropin-releasing hormonea cells; ROS: Reactive oxygen species; GNRH: Gonadotropin-releasing hormone
MicroRNA release from the ovarian tissue in PCOS: Preovulatory GCs exhibit altered microRNA expressions as a result of the LH surge. Numerous ovarian components, including FF and GC, are the subjects of studies on microRNA expression [44]. It is unclear how microRNAs contribute to the pathophysiology of PCOS or follicle formation. A surplus of small follicles is produced in PCOS as a result of gonadotropin-stimulated accelerated early follicular expansion. Inhibin, activins, Growth Differentiation Factors (GDFs) and Bone Morphogenetic Proteins (BMPs) are all members of the TGF-beta superfamily, which is essential for a variety of processes such as follicle development and oocyte maturation [45].
miR-514, miR-144 and miR-513a-3p control several signaling pathways, including TGF-beta, MAPK, oestrogen and oocyte maturation, which are implicated in oocyte growth and maturation in patients with PCOS [46]. The ovaries of PCOS patients exhibit comparatively high levels of miR-27a [29]. The ovaries of individuals with PCOS show differential expression of miR-146a, miR-132, miR-200c, miR-141 and miR-21 [22,31,42]. PCOS patients exhibit downregulated expressions of miR-92a, miR-19b and miR-200a, while women with PCOS show elevated expression levels of miR-21 and miR-146 [47]. The ovary exhibits notable quantities of miR-21, the let-7 family, miR-125b and miR-126 [22,26,28,31].
MicroRNA levels in Granulosa Cells (GC) of PCOS ovaries: About 65% of genes associated with TGF-beta signaling pathways are downregulated in PCOS cumulus cells [46]. Ovarian GC in humans express LH/hCGR and miR-513a-3p. MiR-513a-3p inhibits the expression of LH/hCGR. Follicle maturation in PCOS ovaries may be influenced by miR-513a-3p in response to an aberrant hormonal microenvironment. MiR-508-5p/3p plays a role in focal adhesion, tight junctions, gap junctions and the Extracellular Matrix (ECM)-receptor interaction. Therefore, during the development of oocyte competence, microRNAs may play a role in the cell-to-cell communication system [24]. While miR-142 and miR-33b are increased in GCs of PCOS patients, miR-423 is downregulated. In FF, certain microRNAs are also found to be dysregulated. In GC cultures, these microRNAs have been shown to suppress TGF-κ signaling, apoptosis and cell proliferation, thereby contributing to the pathogenesis of PCOS [48].
In PCOS patients, aberrant folliculogenesis results from increased GC proliferation. Women with PCOS have downregulated miR-145 in their GCs. In GCs, miR-145 mimics increase cell death and inhibit cell growth. Excessive insulin levels increase IRS1, decrease miR-145 expression and promote cell division. MiR-145 may serve as a potential molecular target for addressing GC dysfunction in PCOS [49]. Elevated miR-93 inhibits the GLUT4 receptor, while downregulated miR-92a and miR-92b result in increased expression of IRS-2 proteins. Additionally, elevated miR-133a-3p inhibits the PI3K/AKT pathway. MiR-323-3p is downregulated and decreases the action of Insulin-like Growth Factor-1 (IGF-1). Hyperandrogenism in GCs is caused by the upregulation of miR-93 and miR-21 [10,37].
Upregulated miR-99a acts at the post-transcriptional stage in PCOS to reduce IGF-1R. By inhibiting IGF-1R, miR-99a may reduce proliferation and promote the death of human GCs, which may help to explain the aberrant folliculogenesis seen in PCOS [10]. In GCs, there is an increase in miR-93, which stimulates cell cycle progression and proliferation. Insulin may promote cell proliferation and increase miR-93 expression [10]. GCs have greater levels of miR-3188 and miR-3135b than healthy individuals. The quantity of oocytes, the rate of fertilisation and the rate of cleavage are all related to the expression level of miR-3135b [50].
The target gene for miR-27a expression in GC is SMAD5 and it is linked to IR in women with PCOS. In the GCs of PCOS patients, miR-27a reduces the levels of PPAR-γ [29]. Expressions of miR-27a-3p are dysregulated in the GCs of women with PCOS and are involved in the formation of ovarian follicles. GC apoptosis and early ovarian failure are associated with miR-27a [51]. IL-6 and Tumor Necrosis Factor-alpha (TNF-α) are produced as a result of miR-27a activity. MiR-27a inhibits IL-10, a crucial regulatory cytokine in ovarian function and is implicated in Toll-Like Receptor (TLR) 2/4-activated macrophages [52]. MiR-27a influences the quantity of oestrogen receptors and a higher level of oestrogen receptors in PCOS patients suggests that miR-27a plays a role in the condition [52]. Through the Activator Protein-1 (AP-1) and Nuclear Factor Kappa-B (NF-κB) pathways, miR-301a affects inflammatory cytokines, leading to inflammation associated with obesity [29]. While miR-33b and miR-142 target TGF-R1, miR-423 targets the expression of Suppressor of Mothers Against Decapentaplegic (SMAD) 7 [42]. Seventeen microRNAs in the GCs displayed varying expressions in PCOS. In GCs, MAP3K8 expression is downregulated while miR-509-3p expression is upregulated. By inhibiting MAP3K8 expression, miR-509-3p enhances estradiol secretion. As a result, different microRNAs contribute to the regulation of estradiol synthesis [53]. The RUX2 gene, which regulates steroidogenesis in GCs, is expressed less when miR-320a is downregulated [10].
MicroRNA levels in Theca Cells (TC) of PCOS: PCOS causes the downregulation of miR-92b and miR-92a in TC, which can enhance signal transduction in the androgen and insulin pathways [47]. Consequently, it increases the expression of the CYP17 and GATA6 genes, which in turn boosts androgen production [54]. IRS-2 is the target of downregulated miR-92a and miR-92b in the insulin signaling pathway. This is linked to the IR traits and relative hyperinsulinaemia in PCOS patients. GATA6 and IRS-2 are targets of miR-92a, suggesting a connection between the insulin signaling system and the androgenic pathways [47].
MicroRNAs in Follicular Fluid (FF) in PCOS: In the ovary, miR-125 can regulate androgen activity and influence folliculogenesis [26]. In PCOS, FF exhibits inconsistent levels of miR-320 expression, whereas GCs show elevated levels [34]. Hormonal imbalance is caused by increased levels of miR-18b, miR-146a and miR-135a in FF, which affect dysregulated levels of progesterone, estradiol and testosterone [28,31,36]. Women with fertility issues have higher levels of miR-130b in their FF [29]. MiR-301a may have therapeutic potential as it regulates the expression of genes related to voltage-gated potassium channels in T2DM [29]. FF in PCOS shows elevated expressions of miR-9, miR-18b, miR-32, miR-34c, miR-135a, miR-222, miR-146a and miR-30c. Conversely, lower levels of miR-132 and miR-320 are observed in PCOS patients. Serum insulin levels are positively correlated with miR-222 expression, while serum testosterone levels are inversely correlated with miR-146a expression [32]. According to Sørensen AE et al., in PCOS patients with normal androgen levels, miR-93 and miR-21 are upregulated in GCs but downregulated in FF [32].
In FF, miR-18b affects fertility by promoting progesterone production while inhibiting testosterone and estradiol [28]. MiR-146a inhibits the release of testosterone, estradiol and progesterone. The release of progesterone and testosterone is decreased by miR-135a [31,36]. The FF of PCOS patients contains miR-382-5p, miR-361-3p, miR-199b-5p, miR-381-3p, miR-93-3p, miR-127-3p and miR-425-3p. AMH is associated with miR-199b-5p; age and the Free Androgen Index (FAI) are linked to miR-382-5p, while C-reactive Protein (CRP) is associated with miR-93-3p. Therefore, these microRNAs are valuable for the diagnosis and prognosis of PCOS [28]. In FF and GCs, women with PCOS express higher levels of miR-490-5p, miR-212-3p and miR-4643, while expressing lower levels of miR-647. Thus, microRNAs are essential for the metabolism of DHEA and the production of steroids [55].
MicroRNAs in Adipose Tissue (AT) of PCOS: IR with impaired glucose metabolism in AT is present in about 70% of women with PCOS. Analysis of the signaling components of the IRS/PI3K/AKT pathway in AT revealed that PCOS patients have decreased levels of GLUT4 expression. In AT, there is an upregulation of miR-93, miR-133 and miR-223. While miR-133 and miR-223 target the expression of GLUT4 in cardiomyocytes, miR-93 specifically targets GLUT4 in AT. In AT, HOMA-IR and GLUT4 are associated with miR-93 expression. By directly targeting the 3’ UTR of GLUT4, the overexpression of miR-93 led to the downregulation of GLUT4 gene expression in adipocytes [56].
A new non invasive microRNA profile may differentiate PCOS patients from healthy individuals. Insights into PCOS and potential treatment targets may be revealed by the microRNA-target database [38]. It has been discovered that similar alterations in the ovaries are not reflected in the expression profile of serum microRNAs [41].
Conclusion(s)
Although its exact mechanism is unknown, microRNA plays a significant role in the development of PCOS. Numerous investigations have been conducted to determine the microRNA profile in the reproductive organs, FF and serum of women with PCOS. According to recent research, serum microRNAs may serve as new non invasive biomarkers for the diagnosis and prognosis of PCOS and may pave the way for tailored treatments.
[1]. Sukul S, Ramesh PS, Agasti N, Understanding polycystic ovary syndrome: A multifactorial endocrine disorderJ Clin Diag Res 2021 15(10):BE01-BE05.10.7860/JCDR/2021/51241.15539 [Google Scholar] [CrossRef]
[2]. Sadeghi HM, Adeli I, Calina D, Docea AO, Mousavi T, Daniali M, Polycystic ovary syndrome: A comprehensive review of pathogenesis, management, and drug repurposingInt J Mol Sci 2022 23(2):58310.3390/ijms2302058335054768PMC8775814 [Google Scholar] [CrossRef] [PubMed]
[3]. Sharma P, Jain M, Tripathi M, Sharma M, Halder A, An update on the genetics of polycystic ovary syndromeJ Endocrinol Reprod 2023 27(4):217-24.10.18311/jer/2023/34654 [Google Scholar] [CrossRef]
[4]. Sanchez-Garrido MA, Tena-Sempere M, Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategiesMolecular Metabolism 2020 35:10093710.1016/j.molmet.2020.01.00132244180PMC7115104 [Google Scholar] [CrossRef] [PubMed]
[5]. Combs JC, Hill MJ, Decherney AH, Polycystic ovarian syndrome genetics and epigeneticsClin Obstet Gynecol 2021 64(1):20-25.10.1097/GRF.000000000000058133306497PMC7855879 [Google Scholar] [CrossRef] [PubMed]
[6]. Vitale SG, Fulghesu AM, Mikuš M, Watrowski R, D’Alterio MN, Lin LT, The translational role of mirna in polycystic ovary syndrome: From bench to bedside-A systematic literature reviewBiomedicines 2022 10(8):181610.3390/biomedicines1008181636009364PMC9405312 [Google Scholar] [CrossRef] [PubMed]
[7]. Ranganathan K, Sivasankar V, MicroRNAs - Biology and clinical applicationsJ Oral Maxillofac Pathol 2014 18(2):229-34.10.4103/0973-029X.14076225328304PMC4196292 [Google Scholar] [CrossRef] [PubMed]
[8]. Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, miRNAs as biomarkers in disease: Latest findings regarding their role in diagnosis and prognosisCells 2020 9(2):27610.3390/cells902027631979244PMC7072450 [Google Scholar] [CrossRef] [PubMed]
[9]. Wu Y, Li Q, Zhang R, Dai X, Chen W, Xing D, Circulating microRNAs: Biomarkers of diseaseClinica Chimica Acta 2021 516:46-54.10.1016/j.cca.2021.01.00833485903 [Google Scholar] [CrossRef] [PubMed]
[10]. Nasser JS, Altahoo N, Almosawi S, Alhermi A, Butler AE, The role of MicroRNA, long non-coding RNA and circular RNA in the pathogenesis of polycystic ovary syndrome: A literature reviewInt J Mol Sci 2024 25(2):90310.3390/ijms2502090338255975PMC10815174 [Google Scholar] [CrossRef] [PubMed]
[11]. Kim JY, Kim W, Lee KH, The role of microRNAs in the molecular link between circadian rhythm and autism spectrum disorderAnim Cells Syst (Seoul) 2023 27(1):38-52.10.1080/19768354.2023.218053536860270PMC9970207 [Google Scholar] [CrossRef] [PubMed]
[12]. Gibson E, Mahdy H, Anatomy, Abdomen and Pelvis, Ovary. [Updated 2023 Jul 24]In: StatPearls [Internet] 2024 Jan Treasure Island (FL)StatPearls PublishingAvailable from: https://www.ncbi.nlm.nih.gov/books/NBK545187/ [Google Scholar]
[13]. Sopiarz N, Sparzak PB, Primary ovarian insufficiency. [Updated 2023 Mar 6]In: StatPearls [Internet] 2024 Jan Treasure Island (FL)StatPearls PublishingAvailable from: https://www.ncbi.nlm.nih.gov/books/NBK589674/ [Google Scholar]
[14]. Neto MAC, Ludwin A, Borrell A, Benacerraf B, Dewailly D, da Silva Costa F, Counting ovarian antral follicles by ultrasound: A practical guideUltrasound Obstet Gynecol 2018 51(1):10-20.10.1002/uog.1894529080259 [Google Scholar] [CrossRef] [PubMed]
[15]. Deadmond A, Koch CA, Parry JP, Ovarian reserve testing. [Updated 2022 Dec 21]. In: Feingold KR, Anawalt B, Blackman MR, et al., editorsEndotext [Internet] 2000 South Dartmouth (MA)MDText.com, IncAvailable from: https://www.ncbi.nlm.nih.gov/books/NBK279058/ [Google Scholar]
[16]. Singh S, Pal N, Shubham S, Sarma DK, Verma V, Marotta F, Polycystic ovary syndrome: Etiology, current management, and future therapeuticsJ Clin Med 2023 12(4):145410.3390/jcm1204145436835989PMC9964744 [Google Scholar] [CrossRef] [PubMed]
[17]. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndromeFertil Steril 2004 81:19-25.10.1016/j.fertnstert.2003.10.004 [Google Scholar] [CrossRef]
[18]. Zawadzki JK, Dunaif A, Diagnostic criteria for polycystic ovary syndrome: Towards a rationale approach. In: Dunaif A, Givens JR, Haseltine FP and Merriam GR, EdsPolycystic Ovary Syndrome 1992 BostonBlackwell Scientific Publications:377-384. [Google Scholar]
[19]. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guidelineJ Clin Endocrinol Metab 2006 91:4237-45.10.1210/jc.2006-017816940456 [Google Scholar] [CrossRef] [PubMed]
[20]. O’Brien J, Hayder H, Zayed Y, Peng C, Overview of MicroRNA biogenesis, mechanisms of actions, and circulationFront Endocrinol (Lausanne) 2018 9:40210.3389/fendo.2018.0040230123182PMC6085463 [Google Scholar] [CrossRef] [PubMed]
[21]. Sheu-Gruttadauria J, MacRae IJ, Structural foundations of RNA silencing by argonauteJ Mol Biol 2017 429(17):2619-39.10.1016/j.jmb.2017.07.01828757069PMC5576611 [Google Scholar] [CrossRef] [PubMed]
[22]. Flemr M, Malik R, Franke V, Nejepinska J, Sedlacek R, Vlahovicek K, A retrotransposon-driven dicer isoform directs endogenous small interfering RNA production in mouse oocytesCell 2013 155:807-16.10.1016/j.cell.2013.10.00124209619 [Google Scholar] [CrossRef] [PubMed]
[23]. Yao G, Yin M, Lian J, Tian H, Liu L, Li X, MicroRNA-224 is involved in transforming growth factor-β-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4Mol Endocrinol 2010 24:540-51.10.1210/me.2009-043220118412PMC5419098 [Google Scholar] [CrossRef] [PubMed]
[24]. Troppmann B, Kossack N, Nordhoff V, Schuring AN, Gromoll J, microRNA miR-513a-3p acts as a co-regulator of luteinizing hormone/chorionic gonadotropin receptor gene expression in human granulosa cellsMol Cell Endocrinol 2014 390:65-72.10.1016/j.mce.2014.04.00324747085 [Google Scholar] [CrossRef] [PubMed]
[25]. Wanet A, Tacheny A, Arnould T, Renard P, miR-212/132 expression and functions: Within and beyond the neuronal compartmentNucleic Acids Res 2012 40(11):4742-53.10.1093/nar/gks15122362752PMC3367188 [Google Scholar] [CrossRef] [PubMed]
[26]. Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expressionProc Natl Acad Sci 2014 111:3008-13.10.1073/pnas.131897811124516121PMC3939860 [Google Scholar] [CrossRef] [PubMed]
[27]. Eisenberg I, Nahmias N, Persky MN, Greenfield C, Goldman-Wohl D, Hurwitz A, Elevated circulating Micro-Ribonucleic Acid (miRNA)-200b and miRNA-429 levels in anovulatory womenFertil Steril 2017 107:269-75.10.1016/j.fertnstert.2016.10.00327816236 [Google Scholar] [CrossRef] [PubMed]
[28]. Butler AE, Ramachandran V, Cunningham TK, David R, Gooderham NJ, Benurwar M, Increased MicroRNA levels in women with polycystic ovarian syndrome but without insulin resistance: A pilot prospective studyFront Endocrinol (Lausanne) 2020 11:57135710.3389/fendo.2020.57135733101204PMC7556216 [Google Scholar] [CrossRef] [PubMed]
[29]. Tabrizi ZPF, Miraj S, Tahmasebian S, Ghasemi S, Plasma levels of miR-27a, miR-130b, and miR-301a in polycystic ovary syndromeInt J Mol Cell Med 2020 9(3):198-206. [Google Scholar]
[30]. Murri M, Insenser M, Fernández-Durán E, San-Millán JL, Escobar-Morreale HF, Effects of Polycystic Ovary Syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expressionJ Clin Endocrinol Metab 2013 98:e1835-e1844.10.1210/jc.2013-221824037889 [Google Scholar] [CrossRef] [PubMed]
[31]. Jiang X, Li J, Zhang B, Hu J, Ma J, Cui L, Differential expression profile of plasma exosomal microRNAs in women with polycystic ovary syndromeFertil Steril 2021 115:782-92.10.1016/j.fertnstert.2020.08.01933041053 [Google Scholar] [CrossRef] [PubMed]
[32]. Sørensen AE, Wissing ML, Salö S, Englund AL, Dalgaard LT, MicroRNAs related to Polycystic Ovary Syndrome (PCOS)Genes (Basel) 2014 5:684-708.10.3390/genes503068425158044PMC4198925 [Google Scholar] [CrossRef] [PubMed]
[33]. Song DK, Sung YA, Lee H, The role of serum microRNA-6767-5p as a biomarker for the diagnosis of polycystic ovary syndromePLoS One 2016 11(9):e016375610.1371/journal.pone.016375627677182PMC5038950 [Google Scholar] [CrossRef] [PubMed]
[34]. Rashad NM, Ateya MA, Saraya YS, Elnagar WM, Helal KF, Lashin ME, Association of miRNA− 320 expression level and its target gene endothelin-1 with the susceptibility and clinical features of polycystic ovary syndromeJ Ovarian Res 2019 12:1-0.10.1186/s13048-019-0513-531064393PMC6505291 [Google Scholar] [CrossRef] [PubMed]
[35]. Long W, Zhao C, Ji C, Ding H, Cui Y, Guo X, Characterization of serum microRNAs profile of PCOS and identification of novel non-invasive biomarkersCell Physiol Biochem 2014 33:1304-15.10.1159/00035869824802714 [Google Scholar] [CrossRef] [PubMed]
[36]. Roth LW, McCallie B, Alvero R, Schoolcraft WB, Minjarez D, Katz-Jaffe MG, Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndromeJ Assist Reprod Genet 2014 31(3):355-62.10.1007/s10815-013-0161-424390626PMC3947080 [Google Scholar] [CrossRef] [PubMed]
[37]. Sathyapalan T, David R, Gooderham NJ, Atkin SL, Increased expression of circulating miRNA-93 in women with polycystic ovary syndrome may represent a novel, non-invasive biomarker for diagnosisScientific Reports 2015 5(1):1689010.1038/srep1689026582398PMC4652283 [Google Scholar] [CrossRef] [PubMed]
[38]. Ding CF, Chen WQ, Zhu YT, Bo YL, Hu HM, Zheng RH, Circulating microRNAs in patients with polycystic ovary syndromeHum Fertil 2015 18:22-29.10.3109/14647273.2014.95681125268995 [Google Scholar] [CrossRef] [PubMed]
[39]. Udesen PB, Sørensen AE, Svendsen R, Frisk NL, Hess AL, Aziz M, Circulating miRNAs in women with polycystic ovary syndrome: A longitudinal cohort studyCells 2023 12(7):98310.3390/cells1207098337048055PMC10093401 [Google Scholar] [CrossRef] [PubMed]
[40]. Jiang L, Huang J, Chen Y, Yang Y, Li R, Li Y, Identification of several circulating microRNAs from a genome-wide circulating microRNA expression profile as potential biomarkers for impaired glucose metabolism in polycystic ovarian syndromeEndocrine 2016 53(1):280-90.10.1007/s12020-016-0878-926860517 [Google Scholar] [CrossRef] [PubMed]
[41]. Liu Y, Shi X, Xu B, Wang Z, Chen Y, Deng M, Differential expression of plasma-derived exosomal miRNAs in polycystic ovary syndrome as a circulating biomarkerBiomed Rep 2023 19(6):9210.3892/br.2023.167437901874PMC10603371 [Google Scholar] [CrossRef] [PubMed]
[42]. He T, Liu Y, Jia Y, Wang H, Yang X, Lu G, MicroRNA-141 and MicroRNA-200c are overexpressed in granulosa cells of polycystic ovary syndrome patientsFront Med 2018 5:29910.3389/fmed.2018.0029930420952PMC6215824 [Google Scholar] [CrossRef] [PubMed]
[43]. Hosseini AH, Kohan L, Aledavood A, Rostami S, Association of miR-146a Rs2910164 and miR-222 Rs2858060 polymorphisms with the risk of polycystic ovary syndrome in iranian women: A case–control studyTaiwan J Obstet Gynecol 2017 56:652-56.10.1016/j.tjog.2017.08.01429037553 [Google Scholar] [CrossRef] [PubMed]
[44]. Tu J, Cheung AH, Chan CL, Chan WY, The role of microRNAs in ovarian granulosa cells in health and diseaseFront Endocrinol (Lausanne) 2019 10:17410.3389/fendo.2019.0017430949134PMC6437095 [Google Scholar] [CrossRef] [PubMed]
[45]. Patton BK, Madadi S, Pangas SA, Control of ovarian follicle development by TGFβ family signalingCurr Opin Endocr Metab Res 2021 18:102-10.10.1016/j.coemr.2021.03.00134693075PMC8535782 [Google Scholar] [CrossRef] [PubMed]
[46]. Liu S, Zhang X, Shi C, Lin J, Chen G, Wu B, Altered microRNAs expression profiling in cumulus cells from patients with polycystic ovary syndromeJ Transl Med 2015 13:23810.1186/s12967-015-0605-y26198660PMC4508762 [Google Scholar] [CrossRef] [PubMed]
[47]. Lin L, Du T, Huang J, Huang LL, Yang DZ, Identification of differentially expressed micrornas in the ovary of polycystic ovary syndrome with hyperandrogenism and insulin resistanceChin Med J 2015 128:169-74.10.4103/0366-6999.14918925591557PMC4837833 [Google Scholar] [CrossRef] [PubMed]
[48]. Li Y, Xiang Y, Song Y, Wan L, Yu G, Tan L, Dysregulated miR-142, -33b and -423 in granulosa cells target TGFBR1 and SMAD7: A possible role in polycystic ovary syndromeMol Hum Reprod 2019 25(10):638-46.10.1093/molehr/gaz01430865275 [Google Scholar] [CrossRef] [PubMed]
[49]. Cai G, Ma X, Chen B, Huang Y, Liu S, Yang H, MicroRNA-145 negatively regulates cell proliferation through targeting IRS1 in isolated ovarian granulosa cells from patients with polycystic ovary syndromeReprod Sci 2017 24:902-10.10.1177/193371911667319727799458 [Google Scholar] [CrossRef] [PubMed]
[50]. Hou Y, Wang Y, Xu S, Qi G, Wu X, Bioinformatics identification of microRNAs involved in polycystic ovary syndrome based on microarray dataMol Med Rep 2019 20:281-91.10.3892/mmr.2019.1025331115532PMC6579986 [Google Scholar] [CrossRef] [PubMed]
[51]. Wang M, Sun J, Xu B, Chrusciel M, Gao J, Bazert M, Functional characterization of microRNA-27a-3p expression in human polycystic ovary syndromeEndocrinology 2018 159(1):297-309.10.1210/en.2017-0021929029022 [Google Scholar] [CrossRef] [PubMed]
[52]. He X, Jing Z, Cheng G, MicroRNAs: New regulators of Toll-like receptor signalling pathwaysBiomed Res Int 2014 2014:94516910.1155/2014/94516924772440PMC3977468 [Google Scholar] [CrossRef] [PubMed]
[53]. Chelegahi AM, Ebrahimi SO, Reiisi S, Nezamnia M, A glance into the roles of microRNAs (exosomal and non-exosomal) in polycystic ovary syndromeObstet Gynecol Sci 2024 67(1):30-48.10.5468/ogs.2319338050353PMC10792310 [Google Scholar] [CrossRef] [PubMed]
[54]. Jahromi MS, Tehrani FR, Noroozzadeh M, Zarkesh M, Ghasemi A, Zadeh-Vakili A, Elevated expression of steroidogenesis pathway genes; CYP17, GATA6 and StAR in prenatally androgenized ratsGene 2016 593(1):167-71.10.1016/j.gene.2016.07.06727511375 [Google Scholar] [CrossRef] [PubMed]
[55]. Rad HM, Mowla SJ, Ramazanali F, Valojerdi MR, Characterization of altered microRNAs related to different phenotypes of polycystic ovarian syndrome (PCOS) in serum, follicular fluid, and cumulus cellsTaiwan J Obstet Gynecol 2022 61(5):768-79.10.1016/j.tjog.2022.05.01336088043 [Google Scholar] [CrossRef] [PubMed]
[56]. Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistanceDiabetes 2013 62(7):2278-86.10.2337/db12-096323493574PMC3712080 [Google Scholar] [CrossRef] [PubMed]