The BAL was first introduced as a therapeutic procedure to clear secretions from the alveolar spaces in alveolar proteinosis and bronchial asthma. Reynolds HY later popularised it as a diagnostic procedure [1]. It involves the introduction of a bronchoscope into the tracheobronchial tree and the instillation of 100-200 mL of normal saline, which is then withdrawn in repeated bouts. A return of 30% or more is considered adequate. The lavage allows for the collection of both cellular and non cellular components from the space. Inflammatory cells, malignant cells, as well as microbes and fungi can be identified through microscopic examination. The fluid collected should be sent to the laboratory within an hour and evaluated based on differential cell count, chemical analysis and bacteriological study.
Lung cancer has high rates of morbidity and mortality. Therefore, minimally invasive procedures that are highly sensitive and specific are necessary for diagnosis in these settings. Cytology samples, such as sputum cytology, bronchial brushing, Endobronchial Ultrasound-guided Fine Needle Aspiration (EBUS-FNA) and Transbronchial FNA (TBNA), have been utilised in the evaluation of malignant lung lesions. Bronchoalveolar Lavage Fluid (BALF) cytology is a safe and reliable method for studying the distal airways and alveolar environment for both infectious and non infectious respiratory tract diseases.
This was a retrospective cytohistological study aimed at determining the sensitivity and specificity of BALF cytology in the diagnosis of lung cancer. The study intended to correlate the findings of the histopathological examination with those of BALF cytology to diagnose malignancy, identify the causes of discrepancies in the results and determine the PPV and NPV.
Materials and Methods
This cross-sectional observational study was conducted in the Department of Pathology, King George’s Medical University, Lucknow, Uttar Pradesh, India, as an observational cohort study from September 2022 to September 2023.
Eligibility criteria: All procedurally adequate cases of BAL performed in the Department of Respiratory Medicine and sent for BALF cytology to the Department of Pathology at King George’s Medical University were included in the study, regardless of the clinical indication.
Study Procedure
Histological and cytological diagnosis were retrieved from records. The entire data was anonymised and no patient-related information was recorded. However, the number of slides prepared, adequacy of BALF cytology and the morphological diagnosis provided were recorded. All slides were reviewed.
The standard procedure used for BALF processing in the laboratory: BALF needs to be processed prior to qualitative and quantitative analysis. BALF was processed by a second-year postgraduate student in pathology. The fluid was centrifuged for 10 minutes at 250 g. The supernatant was discarded and the sediment was smeared onto the slides. Alcohol-fixed and air-dried smears were made. Alcohol-fixed smears were stained with Haematoxylin and Eosin (H&E) and air-dried with May-Grünwald-Giemsa (MGG) stain for microscopic examination. The sediment was stored at four degrees Celsius if a further cell block was needed. A BALF sample is deemed adequate if it contains more than 10 alveolar macrophages per 10 high-power fields, according to Chamberlain’s criteria [2].
Statistical Analysis
Data was recorded on Microsoft Excel and sensitivity, specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) were calculated for BALF diagnosis, taking histological diagnosis as the gold standard. Sensitivity was calculated as true positive/(true positive+false negative); specificity as true negative/(true negative+false positive); PPV as true positive/total positive; and NPV as true negative/total negative. The causes of any discordant results were explored.
Results
The BALF sampling was carried out for 261 cases over one year, with a mean age of 56.79 years and a male-to-female ratio of 1.74:1. In the study institute, the adequacy of BAL cytology was 88.12% (230/261). Three cases were suspicious for malignancy [Table/Fig-1a], eight cases were reported as positive for epithelial malignancy [Table/Fig-1b-d], 219 were reported as negative for malignant cells and 31 were deemed inadequate. The results of BALF cytology have been summarised in [Table/Fig-2].
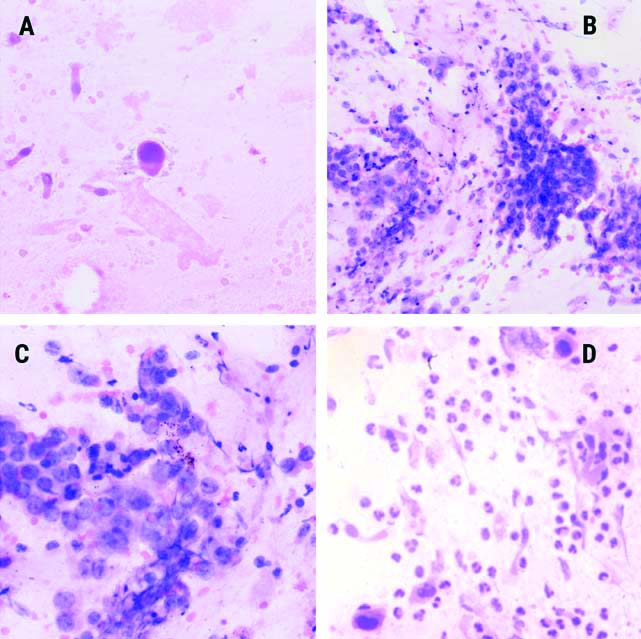
BALF cytology smear showing a) Single atypical cell exhibiting a high nucleocytoplasmic ratio with hyperchromatic nuclei (H&E, x200) Images; b,c) Showing small groups of atypical cells with acinar formation and vesicular nuclei (b- H&E, x200; c- H&E, x400). Malignant epithelial cells with hyperchromatic nuclei and dense eosinophilic cytoplasm consistent with squamous morphology are seen in d (d- H&E, x400).
| Diagnosis | Number of cases | Percentage |
|---|
| Inadequate | 31 | 12% |
| Negative for malignancy | 219 | 84% |
| Suspicious | 3 | 1% |
| Positive for malignancy | 8 | 3% |
| Total | 261 | 100% |
A total of 56 cases out of 261 (21.45%) underwent biopsy. The histological diagnosis has been summarised with the BALF cytology findings of the biopsied cases in [Table/Fig-3]. A total of 32 cases were diagnosed with epithelial malignancy and three were suspicious for malignancy. All three suspicious cases were picked up on BAL; however, surprisingly, only two clear-cut malignant cases were diagnosed on BALF cytology. Five cases that were inconclusive in histology were negative in BALF and 13 cases diagnosed with no evidence of malignancy or nonspecific inflammation were negative in BALF cytology, except for one that was inadequate. During the study, three cases of non epithelial malignancies were diagnosed: two were mesenchymal neoplasms, one fibrous [Table/Fig-4a,b] and one chondroid [Table/Fig-4c,d] and one was a germ cell tumour diagnosed as a germinoma [Table/Fig-5a-d].
Histological diagnosis of samples with group-wise BALF diagnosis for reference.
| Tumour type | No. of cases | Percentage (%) | BALF diagnosis |
|---|
| Inconclusive | 5 | 8.92% | All 5 negatives |
| Negative for malignancy | 13 | 23.21% | 1 inadequate12 negatives |
| Suspicious | 3 | 5.35% | 3 Suspicious |
| Epithelial malignancy:AdenocarcinomaSquamous cell carcinoma (SCC)Not defined | (32)1688 | (57.14%)28.57%14.28%14.28% | 2 Positive30 Negatives |
| Non epithelial malignancy | 3 | 5.35% | 3 Inadequate |
| Total | 56 | | 56 |
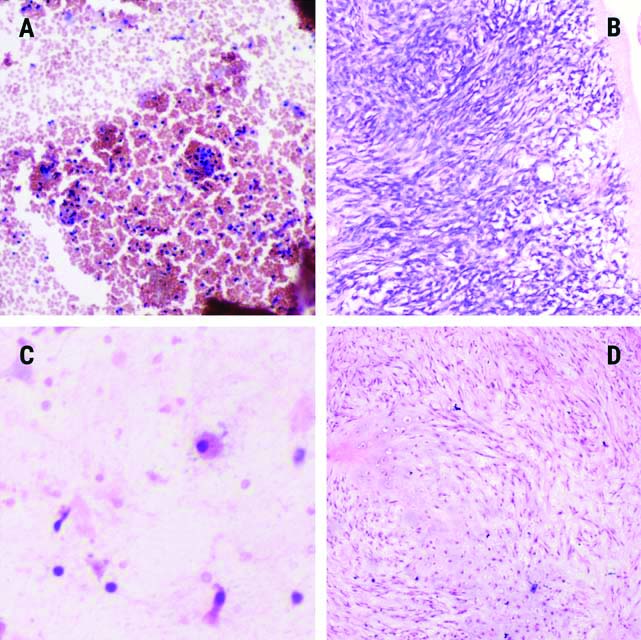
Haematoxylin stained smear of inadequate BALF cytology in ‘a’ with haemorrhage and scattered lymphocytes and in ‘c’ with occasional columnar cell and a single macrophage (a, c; H&E, x200) Image ‘b’ and ‘d’ show counterpart histology of ‘a’ and ‘c’, respectively, with spindle cell tumour in ‘b’ and chondroid tumour in ‘d’ (b and d; H&E, x200).
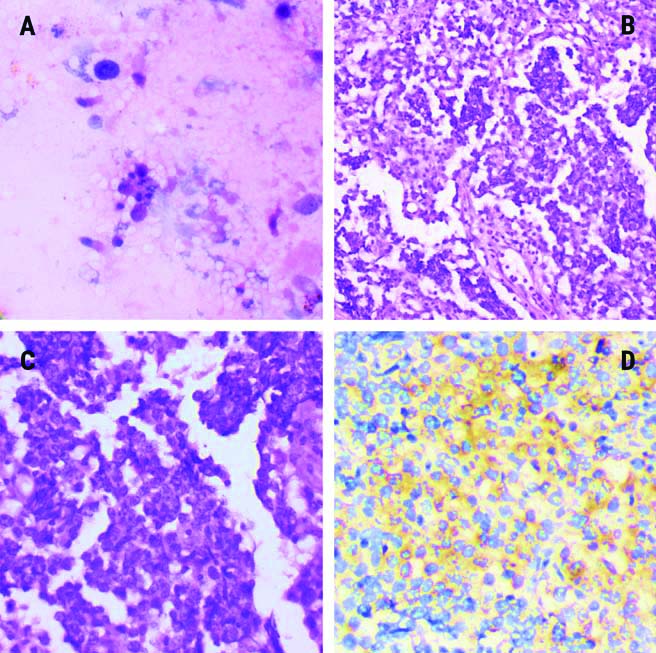
BALF cytology image of haematoxylin and eosin stained smear with occasional alveolar macrophages and scattered columnar cells, no tumour cells were identified in this case (H&E, x200). However, the histology of the same case shows a tumour disposed in a nested pattern with lymphocytic infiltration in the fibrovascular septa. Tumour cells also show Placental-like Alkaline Phosphatase (PLAP) expression as well {(b- H&E, x100; c- H&E, x200; d- 3,3′-Diaminobenzidine (DAB)}.
Sensitivity and specificity were calculated by excluding inadequate BALF cytology samples and inconclusive histology (n=4+5=9). The specificity of BALF cytology with respect to histology was 100%, with a PPV of 100% as well; however, the sensitivity was 14.28% and the NPV was 28.57% [Table/Fig-6]. Efforts were made to identify the causes of the discordance between BALF cytology and histology. For cytological and histological diagnosis, 33 discordant cases were found where there was a histological diagnosis of malignancy. This included 30 epithelial cancers and three non epithelial cancers. All cases of non epithelial malignancies had inadequate BAL cytology [Table/Fig-4]. This may be due to the fact that mesenchymal and non epithelial lesions do not shed cells as readily as epithelial lesions do. Among the 30 epithelial tumours that were not detected by BALF cytology, 18 (60%) were located peripherally. These lesions were beyond the reach of the sampling technique. In five samples, an abundant inflammatory population was observed, which can mask the tumour cell population and hinder proper sampling. The remaining seven cases were reviewed again; they were adequate and negative. No further identifiable cause could be ascertained; hence, it was thought to be due to inadequate sampling or procedural complexities.
Discussion
Lung carcinoma is the leading cause of mortality worldwide and the second most common cancer in both men and women [3]. The increasing incidence of lung cancers has mandated the need for quick and safe investigations to diagnose it as early as possible. Initial screening can be done through bronchial cytology or BAL analysis. Cytological diagnosis requires collaboration between cytopathologists and clinicians for accurate patient management. However, the correlation of cytology with histopathology is of great diagnostic relevance.
A BALF sample is deemed adequate if it contains more than 10 alveolar macrophages per 10 high-power fields, according to Chamberlain’s criteria [2]. However, adequacy can be hampered by cellularity, poor cellular preservation, or haemorrhagic smears. Additionally, the BALF examination has some limitations because it depends on the number of cells exfoliated from the malignant part and a few lung cancers may not exfoliate an adequate number of cells.
In the present study, the age of the patients ranged from 8 to 85 years, with a mean age of 56.8 years. The male-to-female ratio was 1.74:1. Behura A and Rao KM and Choudhury M et al., also revealed a nearly similar age distribution in sampling by BAL [4,5]. On biopsy, the most commonly encountered lung cancer was adenocarcinoma (28.6%), followed by squamous cell carcinoma (14.2%). This is in accordance with data from India’s national cancer registry for 2022 [6].
In the present study, a total of five cases of BAL were correctly diagnosed: two as malignant and three as suspicious. Due to the smaller number of true positive cases, sensitivity was low (14.28%) in the present study [Table/Fig-6]. However, the specificity was found to be 100%, which was higher than the studies by Kedige AR and Dinesh US (88.8%), with diagnostic accuracy being 91.6% [7]. The sensitivity of BALF cytology in lung cancer diagnosis was 39.4% and the specificity was 89.6% in the study by Gaur DS et al., [8]. In the study by Radha S et al., 14.28% (13/91) of cases were diagnosed as malignant, which is similar to our data [9]. The PPV in the present case was 100%, which is also higher than the study by Tayal S and Bhale CP [10].
Sensitivity and specificity of BALF cytology in the study.
| Variables | BALF positive | BALF negative | |
|---|
| Malignant/suspicious diagnosis on histology | TP=5 | FN=30 | PPV=TP/TP+FP100% |
| No malignancy in histology | FP=0 | TN=12 | NPV=TN/FN+TN28.57% |
| Sensitivity=TP/TP+FN=14.28% | Specificity=TN/FP+TN=100% | |
The causes of the high false negative rate (30 cases) or discordant results in the present study were investigated and authors found that this could be attributed to peripherally located tumours (n=18) and an abundant inflammatory population that masks tumour cells (n=5). Furthermore, there have been mentions of a high number of false negative cases, such as those reported by Wongsurakiat P et al., and Gaur DS et al., (13/196 or 6.63%) [8,11]. Technical skill, the amount of lavage drained, or the number of exfoliated tumour cells are also contributing factors. The present data differ from those of Bhat N et al., which reported 31 false positive cases (3.43%). BAL is valuable for diagnosing bacterial pneumonias, tuberculous lesions, fungal infections and malignancies, but its utility in diagnosing cancers is limited [12]. Bronchial brushings have been found to be superior to BALF cytology in cancer diagnosis in lung lesions [8,13].
Although histology is the gold standard for diagnosis, studies of Cytologic-Histologic Correlation (CHC) discrepancies are now recommended worldwide. These studies not only improve pathological practices and patient outcomes but also serve as a means to monitor performance and identify sample types that are susceptible to errors [14]. In studies by Kedige AR and Dinesh US and Gaur DS et al., cytospin combined with filter application for smear preparation significantly improved smear quality [7,8]. However, this method was not available in the present setup, nor in the studies by Radha S et al., and Tayal S and Bhale CP [9,10]. In centres with limited equipment, conventional centrifugation at 250 g for 10 minutes is used for smear preparation. With careful preparation, these smears produce interpretable results with sufficient cytology. Furthermore, techniques such as liquid-based preparations have been investigated, which provide better morphology preservation and transfer of more than 99 percent of cellular yield for EBUS and TBNA [15,16].
Limitation(s)
A major limitation of the present dataset was the lack of histological correlation for a significant portion of the studied population, accounting for 78.54% (205 out of 261 cases). Additionally, the use of conventional techniques for slide preparation was a major limitation of the study. Further investigation into BALF cytology is also necessary.
Conclusion(s)
The present data clearly indicate that while BALF cytology has low sensitivity, it demonstrates high specificity for the diagnosis of lung cancer. Although Bronchoalveolar Lavage (BAL) is a good screening tool, its diagnostic precision can be influenced by the size and location of the tumour. BAL fluid cytology primarily samples the lower respiratory tract, which might not be involved in malignancy, as seen in 18 of the present false-negative cases. Still, the low sensitivity may also be attributed to procedural and interpretative limitations. BALF cytology can serve as an adjunct to bronchial brushings and histology for the diagnosis of lung cancers.