Introduction
The IBS is the most common functional disorder affecting the GI tract and poses a concealed public health threat, with a global prevalence of 3.8 to 9.2% [1,2]. Disruption in the normal interplay of symbiotic GI microbes can trigger a cascade of repercussions, leading to numerous deleterious diseases, with IBS being a major consequence of this microbial imbalance [3]. Patients with IBS, experience various symptoms including abdominal pain, alterations in bowel patterns, difficulties during bowel movements, defecation issues, bloating and feelings of nausea, which may change over time [4,5]. In individuals afflicted with IBS, various mental health conditions can intensify the likelihood of depression and anxiety [6]. The profound interrelation between gut microbes, IBS and the Central Nervous System (CNS) is the underlying cause of the reciprocal occurrence of IBS and psychological distress [7]. The Microbiome-Gut-Brain Axis (MGBA) serves as a communication network linking the GI tract and the brain. The gut microbiota, along with its metabolites, influences plasma neurotransmitter levels and affects the brain through four distinct pathways: neural, endocrine, biochemical and immunological [8].
IBS extends its impact beyond physiological aspects, affecting daily life by impairing attention at work, reducing work productivity, and diminishing overall QoL. The challenges posed by IBS often result in significant loss of work hours, creating a substantial economic burden. The stress associated with IBS further complicates focus at work, leading to increased sick days and disruptions in both work and daily activities. An evaluation considering vital factors such as absenteeism, presenteeism, overall work impairment and activity impairment showed a significant reduction in work hours for individuals suffering from IBS [9]. Given the significant impact of IBS on the physical, social and economic dimensions of public health, the implementation of effective treatment procedures for the management and control of this disorder is urgently needed [10]. The application of probiotic supplementation and faecal microbiota transplantation has been recognised as useful treatments for IBS. Further research and advancement in these treatment approaches are necessary for future progress [11,12].
The novelty of this review lies in its comprehensive evaluation of IBS as a public health threat, explaining its root cause in gut microbial imbalance, focusing on its psychological co-morbidities, exploring work activity impairment and examining potential aspects of future treatments.
Gut Microbiome Insights
Over the last two decades, a prevailing hypothesis has emerged suggesting that the human digestive system, specifically the GI tract, plays a significant role in navigating Multiple Organ Dysfunction Syndrome (MODS) [13]. The GI tract is home to a complex assembly of different microbiomes, including bacteria, archaea, viruses and fungi, along with their collaborative genetic materials. These gut microbes have evolved in conjunction with the host, establishing a mutualistic relationship [14]. The gastrointestinal tract hosts approximately 1014 microbiomes, meaning the number of bacterial cells present in the gut is about ten times greater than the total number of human cells in the entire body, which is estimated to be around 1013. Additionally, the genomic content of these microbiomes is roughly 100 times larger than the total genetic material found in the human genome [15].
Gut Microbe Diversity
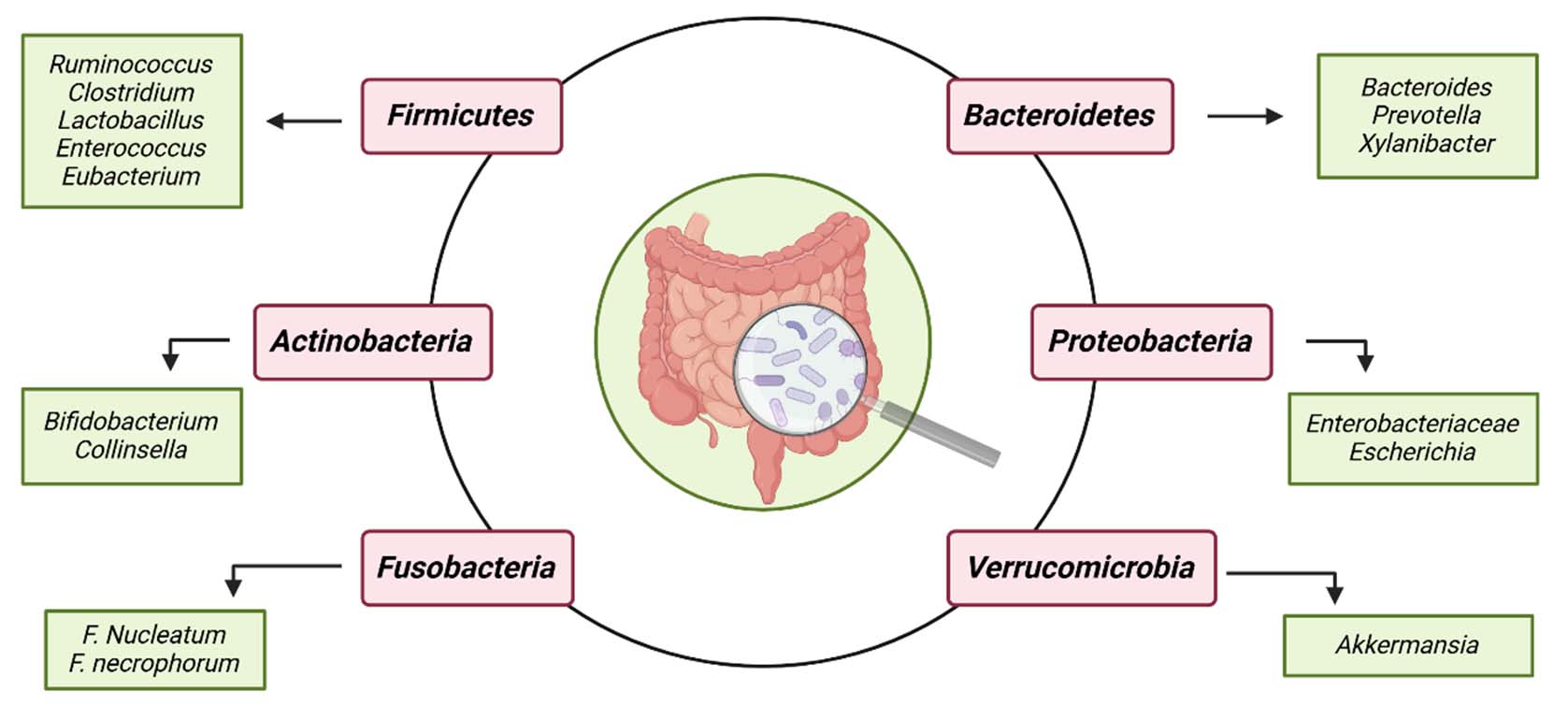
The unique composition of the GI microbial community is influenced by various physicochemical factors, host characteristics and environmental conditions, including pH levels, nutrient availability, host genetics, antibiotic use, ageing, dietary intake patterns and other specific and non specific factors [16]. A study by Hermann-Bank ML et al., reported that Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Verrucomicrobia and Fusobacteria are the most predominant microbial phyla residing in the GI tract [Table/Fig-1], with Firmicutes and Bacteroidetes being the numerically most prevalent [17].
The primary species of the six predominant phyla of gut microbiome. The most common microbial phyla found in the GI tract are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Verrucomicrobia, and Fusobacteria. Created in BioRender.
Gut Microbiota Dysbiosis and Disease
The gut microbiota offers advantages to the host due to its substantial genetic makeup and varied metabolic strategies. Therefore, it is crucial to maintain a proper harmonious interplay among the GI microbes for the wellbeing of the host. However, when the balance and composition of the gut microbiota are disrupted, the gut becomes more prone to pathogenic influences. This imbalance in microbial composition is termed “dysbiosis,” which encompasses alterations in commensal microflora, changes in their functionality, and their metabolic activity [18]. This disparity in microbiome composition can be detrimental to the host and may lead to various diseases such as obesity, diabetes, cancer and autism spectrum disorder, affecting local, systemic, or distal organs [3,19]. Additionally, there is considerable variance in microbial makeup between healthy individuals and those experiencing neurodegenerative conditions, including Multiple Sclerosis (MS), Alzheimer’s Disease (AD), and Parkinson’s Disease (PD) [20].
Modifications in gut microbial composition also play a pivotal role in the development of Inflammatory Bowel Disease (IBD). Research by Wang W et al., confirmed a significant elevation in two important gut microbial phyla, Firmicutes and Actinobacteria (specifically the Bifidobacterium and Lactobacillus groups), as a major change in the gut microbiota of IBD patients [21]. Recent studies have shown that these gut microbial changes play a vital role in the development of IBS [22].
Irritable Bowel Syndrome (IBS)
IBS encompasses a cluster of functional bowel disorders where abdominal soreness or aches are associated with changes in bowel patterns or defecation [1]. The diagnosis of this disorder depends on the assessment of various symptoms, leading to the classification of IBS into different subtypes: IBS-C, where constipation is the predominant symptom; IBS with diarrhoea (IBS-D); mixed IBS (IBS-M); and unsubtyped IBS (IBS-U). IBS-M is also known as altered IBS-A. Symptoms, including abdominal pain, changes in bowel habits, straining during bowel movements, myalgia (muscle pain), bloating and a sense of sickness, vary across patients and may evolve over time. Additionally, patients may experience somatic, visceral and psychiatric co-morbidities. Other factors implicated in the pathogenesis of IBS include disorders in GI motility, visceral hypersensitivity, postinfection reactivity, interactions between the brain and gut, changes in faecal microbiota, food intolerance, malabsorption of carbohydrates and proteins and intestinal inflammation [4,5].
Burden of Irritable Bowel Syndrome
IBS is one of the most prevalent functional GI disorders observed globally [23]. According to the Rome III criteria, the estimated prevalence of IBS in adult populations is 9.2%, while according to the Rome IV criteria, it is 3.8% [2]. An early study on the burden of IBS in Asia reported a median prevalence of 6.5 to 10.1% [24]. In India, the prevalence of the disease ranges from 0.4 to 4.2% [25]. A multicentric study conducted by the Indian Society of Gastroenterology found that middle-aged males were the most common to report IBS-related complications in India [26]. A study involving a rural community in North India reported an IBS prevalence of 4%, whereas a study involving urban residents in western India reported approximately 7.5% prevalence of IBS [27]. In Southeast and Middle Eastern Asia, the disease prevalence is around 0.7%. This lower prevalence in South Asian countries may be attributed to several factors: i) a significant portion of the population resides in rural areas; ii) lifestyle changes associated with rapid urban development; and iii) cultural, religious and dietary practices [28].
Gut Microbes’ Alteration and IBS
IBS can be influenced by the overgrowth or undergrowth of commensals, which constitutes one of the primary reasons for the condition [Table/Fig-2] [29-32]. In a study involving 25 IBS patients and 25 matched controls, Si JM et al., found a notable reduction in faecal Bifidobacterium and a significant increase in Enterobacteriaceae in individuals with IBS compared to the levels observed in healthy controls [29].
Alteration in the composition of Gut microbiome in IBS patients [29-32].
| Study | Patient number | Dysbiosis in patients |
|---|
| Si JM et al., (2004) [29] | IBS-25Control-25 | Bifidobacterium (←)Enterobacteriaceae (←) |
| Malinen E et al., (2005) [30] | IBS-27Control-22 | Lactobacillus spp. (↓)Veillonella spp. (←) |
| Rajilić-Stojanović M et al., (2011) [31] | IBS-62Control-46 | Firmicutes toBacteroidetes ratio (←) |
| Jeffery IB et al., (2020) [32] | IBS-80Control-65 | R. gnavus, Lachnospiraceae spp (←)B. intestinihominis, C. catus (↓) |
Multiple research studies indicate that people with and without IBS have observable differences in their gut microbial compositions. (IBS: Irritable bowel syndrome, (←))
Malinen E et al., found a decreased number of Lactobacillus spp. in diarrhoea-predominant IBS samples, while constipation-predominant IBS samples showed elevated quantities of Veillonella spp. among the IBS patients and the control group [30]. Another investigation by Rajilić-Stojanović M et al., reinforced the clear divergence in the composition of GI microbiota between individuals with IBS and those without the condition. The study highlighted an increased ratio of Firmicutes to Bacteroidetes in IBS patients [31]. When Jeffery IB et al., conducted a species-level analysis, they discovered significant differences in the abundance of microorganisms. Specifically, Ruminococcusgnavus and Lachnospiraceae spp. exhibited significantly higher prevalence in patients with IBS, while taxa such as Barnesiella intestinihominis and Coprococcus catus were found to be significantly less abundant in IBS patients compared to the control group [32].
While the aforementioned research suggests notable changes in gut microbiota in individuals with IBS, there are also several other studies that have not observed any significant alterations in the normal gut microbial composition among IBS patients [33].
GUT-IBS-Psychological Co-Morbidities
Anxiety, depression and other widespread mental health issues are now predominant worldwide and are crucial contributors to disability and suicide [6]. Among various mental health conditions, Major Depressive Disorder (MDD) and anxiety disorders are highlighted as the most prevalent. Persistent depression or low mood, diminished pleasure or interest, and other associated psychological and cognitive symptoms are the main characteristics of MDD. This condition also encompasses feelings of dread, restlessness and anxiety. Although there are several pharmacological treatments for these disorders, patients often opt out or discontinue treatment due to side-effects, social stigma, or treatment resistance, leaving them without effective strategies for improving their condition [34].
In the realm of gut-brain psychology, gut microbiota plays a significant role in the gut-brain network. The development of gut microbiota is closely aligned with that of the brain and mind [35]. Notably, IBS is one of the most common disorders of gut-brain interaction, affecting an estimated one-tenth of the global population [26]. It is associated with various mental health disorders in such a way that it elevates the risk of depression and anxiety in individuals with IBS [6].
Microbiome-Gut-Brain Axis (MGBA)
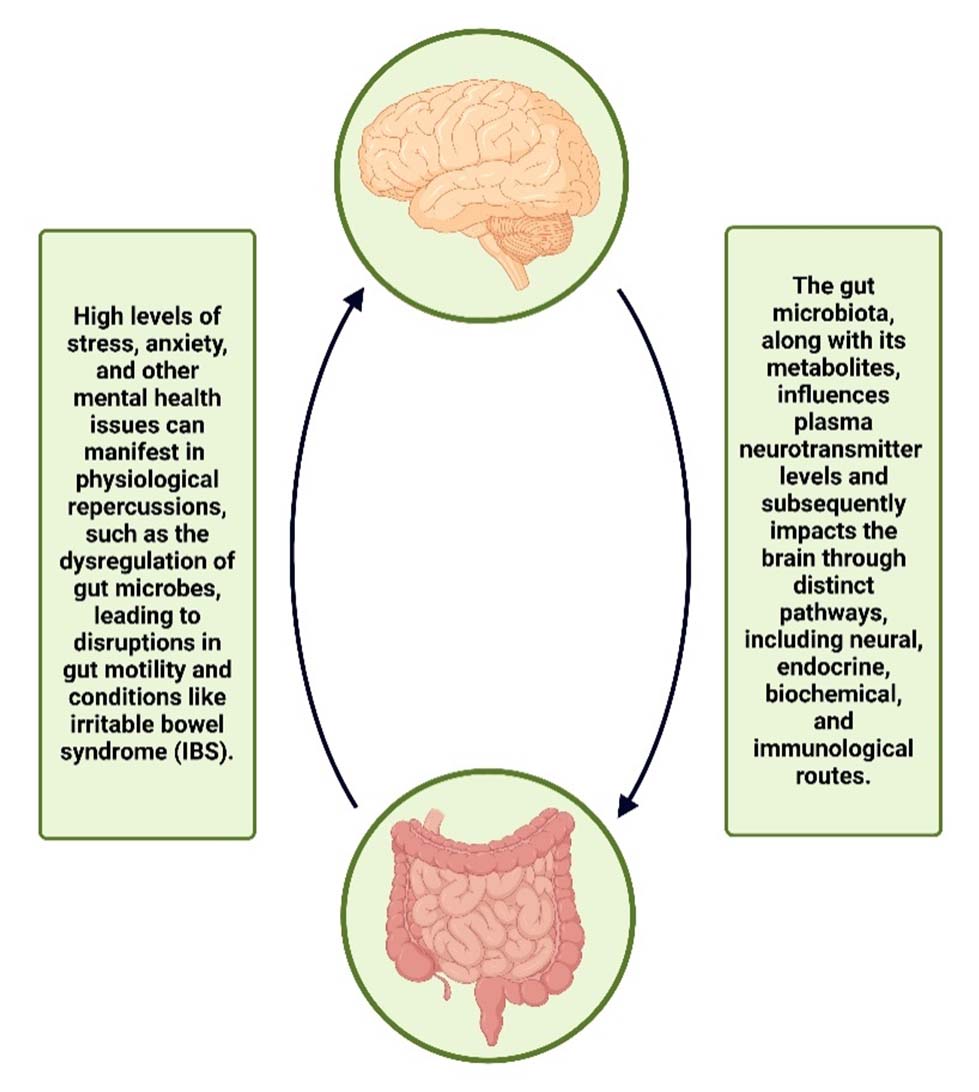
There has been increasing emphasis on the importance of bidirectional relation between CNS and GI microbiome [Table/Fig-3], commonly referred to as the MGBA. The research field of the “gut-brain axis” has gained significant attention recently. This axis serves as a transmission network that integrates hormonal, neural and immunological signals between the GI tract and the brain, offering a prospective pathway for gut microbiota and its metabolites to influence the brain. There are four different pathways (neural, endocrine, biochemical and immunological) through which the gut microbiome regulates the gut-brain axis both directly and indirectly [7,8].
Bidirectional relation of gut-brain-axis. The brain is influenced by the gut microbiota and its metabolites, and dysregulation of gut microbes is a consequence of mental health problems. This indicates the reciprocal relationship between the Central Nervous System (CNS) and the Gastrointestinal (GI) microbiome.
Created in BioRender
Gut Microbiome and Neurotransmitter Alliance and Psychology
The co-occurring condition of IBS and psychological stress is also mediated by the MGBA. In a research study exploring the association between plasma neurotransmitter levels (serotonin, norepinephrine) and gut microbiota, scientists found neurotransmitters to be key participants in the gut-brain axis and contributors to emotional discomfort and IBS. This study reported a positive correlation between serotonin and norepinephrine with the abundance of Proteobacteria and Bacteroidetes, respectively [36].
Serotonin, one of the most important neurotransmitters in the brain-gut axis, is primarily synthesised in the gut. Several experiments have demonstrated that bacteria, including Escherichia, Lactobacillus, and Klebsiellapneumoniae, possess the ability to produce serotonin. Some literature indicates that neurotransmitters, such as dopamine and norepinephrine, can also be synthesised by bacteria like Lactobacillus, Bacillus and Klebsiella [37].
Gut Microbes’ Alteration and Emotional Distress
The influence of the gut microbiome extends to numerous fundamental physiological processes and mental conditions, participating in the pathophysiology of various mental and nervous system-related disorders. Thus, alterations in gut microbiota composition are believed to play a significant role in disorders like depression, stress, anxiety and even autism [38]. This interplay, between disturbances in the microbial community of the GI tract and the worsening of psychological disorders has been demonstrated in several studies earlier [Table/Fig-4] [39-42].
Alteration in the composition of gut microbiome in patients with psychological disorder. According to numerous research studies, there are discernible variations in the gut microbial compositions of individuals with and without psychological disorders such as MDD or BD.
| Study | Patient number | Dysbiosis in patients withpsychological disorder |
|---|
| Jiang H et al., (2015) [39] | MDD-46 Control-30 | Firmicutes (↓)Bacteroidetes,Actinobacteria,Proteobacteria (←) |
| Huang Y et al., (2018) [40] | MDD-27 Control-27 | Firmicutes (↓) |
| Chen JJ et al., (2018) [41] | Female: MDD-24Control-24Male: MDD-20Control-20 | Female: Actinobacteria (←)Male: Bacteroidetes (↓) |
| Painold A et al., (2019) [42] | BD-32 Control-10 | Actinobacteria, Coriobacteriia (←)Faecalibacterium, Ruminococcaceae (↓) |
(MDD: Major depressive disorder; BD: Bipolar disorder (←); Higher prevalence than control, (↓): Lower prevalence than control)
According to the study by Jiang H et al., Bacteroidetes, Actinobacteria, and Proteobacteria were more abundant in individuals with active MDD or those previously treated for the same, whereas Firmicutes were significantly reduced in those patients [39]. Another study by Huang Y et al., also supported this decrease in Firmicutes in MDD patients [40].
Chen JJ et al., highlighted gender-based differences in gut microbiota compositions among individuals with MDD and healthy controls, observing elevated levels of Actinobacteria in females and lower numbers of Bacteroidetes in males [41]. In a study involving patients with Bipolar Disorder (BD), Painold A et al., reported an increase in the number of Actinobacteria and Coriobacteriia, along with a reduction in Faecalibacterium and Ruminococcaceae [42]. The composition of microbiota differs between those with and without depression. In individuals with IBS, the normal microbial makeup also varies based on the presence or absence of psychological co-morbidities [43].
Psychological Impact of IBS
Constant worry: Always being worried about when and where stomach troubles will occur. It’s like having a nagging feeling of unease all the time.
Feeling down: IBS can lead individuals to feel sad and hopeless because it keeps recurring. It’s like a cloud that won’t go away [44].
Feeling alone and left out: Sometimes, IBS may cause individuals to want to stay home and avoid seeing friends. It feels like being excluded from social activities they used to enjoy.
Work struggles: IBS can make it difficult to concentrate at work and individuals might miss days due to not feeling well. This can lead to feelings of inadequacy.
Low self-esteem: IBS can change an individual’s body and make them feel self-conscious. It’s like not feeling good about the way they look.
Keeping a secret: Some people with IBS feel the need to keep it a secret due to embarrassment. It’s like having something they are hiding from others.
Frustration with treatments: Trying different treatments, but nothing seems to work. It’s like attempting to fix a problem that keeps recurring, which can be very frustrating.
Worrying about health: IBS can lead individuals to worry excessively about their health, even when there is no serious danger. It’s like always thinking that something bad might happen [45].
In simple terms, IBS can be mentally challenging. It affects how individuals feel about themselves, their relationships, and their daily lives. Having support and understanding from others can make a significant difference for someone dealing with IBS.
IBS and Social Impact
The Social Impact of IBS on Man-Hour Loss in a more Humanised Manner
Absenteeism and wellbeing: IBS can sometimes lead individuals to take more sick days from work. This isn’t just about numbers; it’s about people needing time off to manage their health and well-being.
Daily challenges: Frequent discomfort and the need for bathroom breaks can disrupt daily tasks at work. Consider how this could affect your own productivity and how it might impact someone you know.
Navigating relationships: The embarrassment and hesitancy to discuss IBS can make workplace relationships more challenging. Imagine how it might feel to have to keep something so personal hidden.
Mental health: Managing IBS can be stressful, and the resulting anxiety can make it difficult to focus at work [46]. We all know how stress can impact our ability to function effectively.
Balancing health: Medical appointments and treatments may require time away from work. It’s a balancing act between health needs and work responsibilities that many individuals face.
Flexibility and understanding: Some people with IBS may need flexible work arrangements. Consider how accommodating such needs might be a way to support your colleagues’ wellbeing.
Creating a supportive environment: Employers may need to make accommodations. This is about creating an inclusive and supportive work environment for everyone.
In essence, IBS touches the lives of real people, and its impact on man-hour loss is a reflects the everyday challenges they face [47]. Empathy, understanding, and effective collaboration in the workplace can make a significant difference in the lives of those dealing with IBS.
Impression of IBS Daily Activities and Work
The quality of life, especially work life and daily activities, is disrupted for people with IBS. This assessment of work hours lost due to IBS considers four key factors: i) Absenteeism, indicating the percentage of work time lost because of IBS; ii) Presenteeism, reflecting the percentage of impairment faced while at work; iii) Overall work impairment, representing the total productivity loss in percentage; and iv) Activity impairment, which measures the percentage of impairment in daily activities [9].
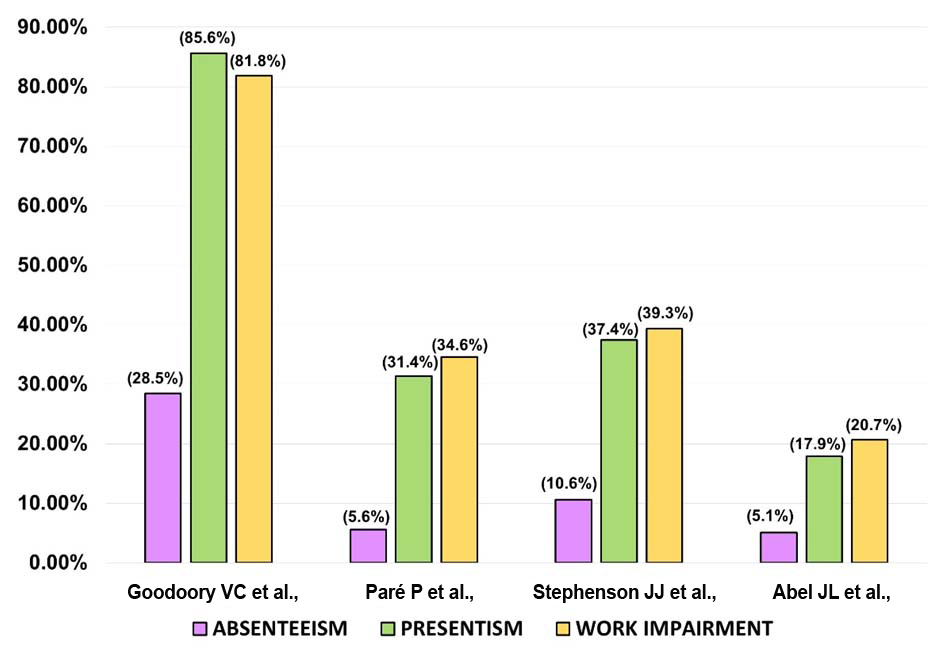
A prior study by Dean BB et al., demonstrated a 21% reduction in work productivity, equating to less than four days of work in a five-day workweek. Several other studies have also reported overall work loss in IBS patients [48]. Multiple investigations show that respondents with IBS experience lower productivity at work (presenteeism, absenteeism, and overall work impairment) [Table/Fig-5] [49-52].
Work loss (%) due to IBS in different studies. Several studies show that persons with Irritable bowel syndrome have reduced work effectiveness (absenteeism, presenteeism, and overall work impairment).
The Economic Impact of Irritable Bowel Syndrome (IBS) in a Simpler, More Relatable Way
Medical bills: IBS can lead to numerous doctor visits, tests, and medications. All these medical expenses can add up and lighten their wallet.
Missed work: IBS might force individuals to miss work or make it difficult to perform their job. When they miss work, they might not earn as much as they’d like.
Special foods: Some people with IBS need to buy special foods that can be more expensive than regular ones.
Medicine costs: One might need to purchase medicine to feel better, which can also be costly.
Travel expenses: Frequent doctor visits or the need to find restrooms while out can increase travel expenses.
Missing out: IBS can make individuals skip social events and enjoyable activities, leading to feelings of missing out on good times [53].
Feeling down: Dealing with IBS can be tough, and they might need to see a therapist or counselor, which costs money.
Trying different treatments: Some people try various treatments, which can also be expensive.
Insurance costs: Individuals might have to pay more for health insurance or face higher bills because of IBS.
Less money: All of these costs can accumulate, leading to less money in their pockets.
In simple terms, IBS can affect an individual’s finances in various ways, from medical bills to missed work opportunities. It’s essential to plan and budget carefully to manage these financial challenges [54].
Treatment and Future Perspective
Recent clinical trials have established the efficacy and safety of multiple treatments for IBS. The first-line treatment for IBS includes lifestyle and dietary changes. Medications to address associated symptoms incorporate antispasmodics and peppermint for abdominal pain, as well as loperamide and laxatives for diarrhoea and constipation, respectively. Central neuromodulators, preferably tricyclic antidepressants, can also be used [10]. For patients with constipation-predominant IBS, linaclotide has been found to reduce both the frequency of bowel movements and abdominal pain. Other medications that provide targeted treatment opportunities for IBS patients include rifaximin, which targets gut microbiota and asimadoline and eluxadoline, which target opioid receptors [55]. In a comparative study with a placebo, Abel JL et al., reported significant improvement in health-related QoL in IBS patients [52].
Over three decades, Tricyclic Antidepressants (TCAs), such as amitriptyline, nortriptyline and desipramine, have been used to treat IBS and other functional bowel disorders. However, the adverse side-effects linked to their use might constrain their efficacy. Another treatment approach can be serotonin reuptake inhibitors, which include citalopram, paroxetine and fluoxetine. In some studies, these have been reported to reduce bloating, abdominal pain and overall wellbeing, regardless of whether the patients have psychological issues. They are also thought to affect the enteric nervous system. Venlafaxine and duloxetine, examples of selective serotonin and norepinephrine reuptake inhibitors, may also be effective in treating IBS-related pain [56].
According to several studies, supplemental probiotic therapy proves efficacious in alleviating abdominal pain associated with paediatric IBS. Probiotics have also been found to reduce mental health problems like anxiety and depression in various studies. An additional pilot investigation conducted by Pinto-Sanchez MI et al., revealed that the probiotic Bifidobacterium longum not only decreases depression scores but also induces changes in brain activity among individuals diagnosed with IBS [11]. Apart from probiotics, various approaches such as prebiotics, postbiotics and the administration of antibiotics also show promise as techniques for modulating gut microbiota, displaying notable effectiveness in treating various diseases [54].
Another emerging treatment process, FMT, involves transferring microorganisms from a healthy donor to a diseased individual. Unlike medications, FMT comprises intricate combinations of live organisms that need to interact with the microorganisms and immune system of sick patients [12]. A study conducted by El-Salhy M et al., provides backing for the effectiveness of FMT as a treatment for individuals with IBS [57]. However, in contrast, a study by Abdelghafar YA et al., concludes that FMT is not a viable treatment option for IBS [58].
As connections between gut microbiota and depression have been noted in recent years, FMT has also been applied in the treatment of mental health issues. Research on FMT involving two patients as an adjunctive therapy for depression reveals an enhancement in depressive symptoms four weeks post-transplantation [59]. In another study involving a patient with treatment-resistant bipolar disorder, FMT was also identified as a beneficial treatment [60].
Conclusion(s)
To sum up, the complex relationship that exists between GI bacteria, mental health and day-to-day functioning makes IBS a major global public health issue. The multifaceted connection between the gut, microbes and the CNS is highlighted by the noticeable dysbiosis in gut microbiota among individuals who have both IBS and co-occurring mental health disorders. IBS has a widespread negative influence on productivity at work and overall QoL, which calls for immediate attention and effective interventions.
There is hope for future treatments with promising modalities such as probiotics, prebiotics, FMT and medications, all of which require further investigation and study. Given the significant social and financial consequences of challenges related to IBS, it is imperative that this issue be addressed as promptly as possible. It is believed that this comprehensive review supports a multidisciplinary approach by recognising the microbial origin of IBS, examining psychological co-morbidities, and highlighting the critical need for effective interventions to be implemented quickly in order to minimise the burden on both individuals and society.
(MDD: Major depressive disorder; BD: Bipolar disorder (←); Higher prevalence than control, (↓): Lower prevalence than control)
[1]. Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA, Functional bowel disorders and functional abdominal painGut 1999 45(Suppl 2):II43-II47.10.1136/gut.45.2008.ii4310457044PMC1766683 [Google Scholar] [CrossRef] [PubMed]
[2]. Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC, Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysisLancet Gastroenterol Hepatol 2020 5(10):908-17.10.1016/S2468-1253(20)30217-X32702295 [Google Scholar] [CrossRef] [PubMed]
[3]. Kho ZY, Lal SK, The human gut microbiome - A potential controller of wellness and diseaseFront Microbiol 2018 9:183510.3389/fmicb.2018.0183530154767PMC6102370 [Google Scholar] [CrossRef] [PubMed]
[4]. Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Irritable bowel syndromeNat Rev Dis Primers 2016 2:1601410.1038/nrdp.2016.1427159638PMC5001845 [Google Scholar] [CrossRef] [PubMed]
[5]. Saha L, Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicineWorld J Gastroenterol 2014 20(22):6759-73.10.3748/wjg.v20.i22.675924944467PMC4051916 [Google Scholar] [CrossRef] [PubMed]
[6]. Staudacher HM, Black CJ, Teasdale SB, Mikocka-Walus A, Keefer L, Irritable bowel syndrome and mental health comorbidity - Approach to multidisciplinary managementNat Rev Gastroenterol Hepatol 2023 20(9):582-96.10.1038/s41575-023-00794-z37268741PMC10237074 [Google Scholar] [CrossRef] [PubMed]
[7]. Collins SM, Surette M, Bercik P, The interplay between the intestinal microbiota and the brainNat Rev Microbiol 2012 10(11):735-42.10.1038/nrmicro287623000955 [Google Scholar] [CrossRef] [PubMed]
[8]. Appleton J, The gut-brain axis: Influence of microbiota on mood and mental healthIntegr Med (Encinitas) 2018 17(4):28-32. [Google Scholar]
[9]. Frändemark Å, Törnblom H, Jakobsson S, Simrén M, Work productivity and activity impairment in Irritable Bowel Syndrome (IBS): A multifaceted problemAm J Gastroenterol 2018 113(10):1540-49.10.1038/s41395-018-0262-x30254230 [Google Scholar] [CrossRef] [PubMed]
[10]. Black CJ, Ford AC, Best management of irritable bowel syndromeFrontline Gastroenterol 2020 12(4):303-15.Published 2020 May 2810.1136/flgastro-2019-10129834249316PMC8231425 [Google Scholar] [CrossRef] [PubMed]
[11]. Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndromeGastroenterology 2017 153(2):448-459.e8.10.1053/j.gastro.2017.05.00328483500 [Google Scholar] [CrossRef] [PubMed]
[12]. Chu ND, Crothers JW, Nguyen LTT, Kearney SM, Smith MB, Kassam Z, Dynamic colonization of microbes and their functions after fecal microbiota transplantation for inflammatory bowel diseasemBio 2021 12(4):e009752110.1128/mBio.00975-2134281401PMC8406238 [Google Scholar] [CrossRef] [PubMed]
[13]. Clark JA, Coopersmith CM, Intestinal crosstalk: A new paradigm for understanding the gut as the “motor” of critical illnessShock 2007 28(4):384-93.10.1097/shk.0b013e31805569df17577136PMC2084394 [Google Scholar] [CrossRef] [PubMed]
[14]. Sevcikova A, Izoldova N, Stevurkova V, Kasperova B, Chovanec M, Ciernikova S, The impact of the microbiome on resistance to cancer treatment with chemotherapeutic agents and immunotherapyInt J Mol Sci 2022 23(1):48810.3390/ijms2301048835008915PMC8745082 [Google Scholar] [CrossRef] [PubMed]
[15]. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Metagenomic analysis of the human distal gut microbiomeScience 2006 312(5778):1355-59.10.1126/science.112423416741115PMC3027896 [Google Scholar] [CrossRef] [PubMed]
[16]. Hasan N, Yang H, Factors affecting the composition of the gut microbiota, and its modulationPeer J 2019 7:e750210.7717/peerj.750231440436PMC6699480 [Google Scholar] [CrossRef] [PubMed]
[17]. Hermann-Bank ML, Skovgaard K, Stockmarr A, Larsen N, Mølbak L, The gut microbiotassay: A high-throughput qPCR approach combinable with next generation sequencing to study gut microbial diversityBMC Genomics 2013 14:78810.1186/1471-2164-14-78824225361 [Google Scholar] [CrossRef] [PubMed]
[18]. Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ, Dysbiosis of the gut microbiota in diseaseMicrob Ecol Health Dis 2015 26:2619110.3402/mehd.v26.2619125651997PMC4315779 [Google Scholar] [CrossRef] [PubMed]
[19]. DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E, Current understanding of dysbiosis in disease in human and animal modelsInflamm Bowel Dis 2016 22(5):1137-50.10.1097/MIB.000000000000075027070911PMC4838534 [Google Scholar] [CrossRef] [PubMed]
[20]. Rutsch A, Kantsjö JB, Ronchi F, The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathologyFront Immunol 2020 11:60417910.3389/fimmu.2020.60417933362788PMC7758428 [Google Scholar] [CrossRef] [PubMed]
[21]. Wang W, Chen L, Zhou R, Wang X, Song L, Huang S, Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel diseaseJ Clin Microbiol 2014 52(2):398-406.10.1128/JCM.01500-1324478468PMC3911339 [Google Scholar] [CrossRef] [PubMed]
[22]. Menees S, Chey W, The gut microbiome and irritable bowel syndromeF1000Res 2018 7F1000 Faculty Rev-102910.12688/f1000research.14592.130026921PMC6039952 [Google Scholar] [CrossRef] [PubMed]
[23]. Vahedi H, Ansari R, Mir-Nasseri M, Jafari E, Irritable bowel syndrome: A review articleMiddle East J Dig Dis 2010 2(2):66-77. [Google Scholar]
[24]. Chang FY, Lu CL, Chen TS, The current prevalence of irritable bowel syndrome in AsiaJ Neurogastroenterol Motil 2010 16(4):389-400.10.5056/jnm.2010.16.4.38921103420PMC2978391 [Google Scholar] [CrossRef] [PubMed]
[25]. Ghoshal UC, Sachdeva S, Pratap N, Karyampudi A, Mustafa U, Abraham P, Indian consensus statements on irritable bowel syndrome in adults: A guideline by the Indian Neurogastroenterology and Motility Association and jointly supported by the Indian Society of GastroenterologyIndian J Gastroenterol 2023 42(2):249-73.10.1007/s12664-022-01333-536961659PMC10036984 [Google Scholar] [CrossRef] [PubMed]
[26]. Ghoshal UC, Abraham P, Bhatt C, Choudhuri G, Bhatia SJ, Shenoy KT, Epidemiological and clinical profile of irritable bowel syndrome in India: Report of the Indian Society of Gastroenterology Task ForceIndian J Gastroenterol 2008 27(1):22-28. [Google Scholar]
[27]. Black CJ, Ford AC, Global burden of irritable bowel syndrome: Trends, predictions and risk factorsNat Rev Gastroenterol Hepatol 2020 17(8):473-86.10.1038/s41575-020-0286-832296140 [Google Scholar] [CrossRef] [PubMed]
[28]. Rahman MM, Mahadeva S, Ghoshal UC, Epidemiological and clinical perspectives on irritable bowel syndrome in India, Bangladesh and Malaysia: A reviewWorld J Gastroenterol 2017 23(37):6788-801.10.3748/wjg.v23.i37.678829085223PMC5645613 [Google Scholar] [CrossRef] [PubMed]
[29]. Si JM, Yu YC, Fan YJ, Chen SJ, Intestinal microecology and quality of life in irritable bowel syndrome patientsWorld J Gastroenterol 2004 10(12):1802-05.10.3748/wjg.v10.i12.180215188510PMC4572273 [Google Scholar] [CrossRef] [PubMed]
[30]. Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCRAm J Gastroenterol 2005 100(2):373-82.10.1111/j.1572-0241.2005.40312.x15667495 [Google Scholar] [CrossRef] [PubMed]
[31]. Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndromeGastroenterology 2011 141(5):1792-801.10.1053/j.gastro.2011.07.04321820992 [Google Scholar] [CrossRef]
[32]. Jeffery IB, Das A, O’Herlihy E, Coughlan S, Cisek K, Moore M, Differences in fecal microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorptionGastroenterology 2020 158(4):1016-1028.e8.10.1053/j.gastro.2019.11.30131843589 [Google Scholar] [CrossRef] [PubMed]
[33]. Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, Gut microbiota in patients with irritable bowel syndrome-A systematic reviewGastroenterology 2019 157(1):97-108.10.1053/j.gastro.2019.03.04930940523 [Google Scholar] [CrossRef] [PubMed]
[34]. Ter Meulen WG, Draisma S, van Hemert AM, Schoevers RA, Kupka RW, Beekman ATF, Depressive and anxiety disorders in concert-A synthesis of findings on comorbidity in the NESDA studyJ Affect Disord 2021 284:85-97.10.1016/j.jad.2021.02.00433588240 [Google Scholar] [CrossRef] [PubMed]
[35]. Dash S, Syed YA, Khan MR, Understanding the role of the gut microbiome in brain development and its association with neurodevelopmental psychiatric disordersFront Cell Dev Biol 2022 10:88054410.3389/fcell.2022.88054435493075PMC9048050 [Google Scholar] [CrossRef] [PubMed]
[36]. Barandouzi ZA, Lee J, del Carmen Rosas M, Chen J, Henderson WA, Starkweather AR, Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndromeSci Rep 2022 12:164810.1038/s41598-022-05756-035102266PMC8803858 [Google Scholar] [CrossRef] [PubMed]
[37]. Averina OV, Danilenko VN, Human intestinal microbiota: Role in development and functioning of the nervous systemMicrobiol 2017 86(1):05-24.10.1134/S0026261717010040 [Google Scholar] [CrossRef]
[38]. MacQueen G, Surette M, Moayyedi P, The gut microbiota and psychiatric illnessJ Psychiatry Neurosci 2017 42(2):75-77.10.1503/jpn.17002828245172PMC5373703 [Google Scholar] [CrossRef] [PubMed]
[39]. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Altered fecal microbiota composition in patients with major depressive disorderBrain Behav Immun 2015 48:186-94.10.1016/j.bbi.2015.03.01625882912 [Google Scholar] [CrossRef] [PubMed]
[40]. Huang Y, Shi X, Li Z, Shen Y, Shi X, Wang L, Possible association of Firmicutes in the gut microbiota of patients with major depressive disorderNeuropsychiatr Dis Treat 2018 14:3329-37.10.2147/NDT.S18834030584306PMC6284853 [Google Scholar] [CrossRef] [PubMed]
[41]. Chen JJ, Zheng P, Liu YY, Zhong XG, Wang HY, Guo YJ, Sex differences in gut microbiota in patients with major depressive disorderNeuropsychiatr Dis Treat 2018 14:647-55.10.2147/NDT.S15932229520144PMC5833751 [Google Scholar] [CrossRef] [PubMed]
[42]. Painold A, Mörkl S, Kashofer K, Halwachs B, Dalkner N, Bengesser S, A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episodeBipolar Disord 2019 21(1):40-49.10.1111/bdi.1268230051546PMC6585963 [Google Scholar] [CrossRef] [PubMed]
[43]. Simpson CA, Mu A, Haslam N, Schwartz OS, Simmons JG, Feeling down? A systematic review of the gut microbiota in anxiety/depression and irritable bowel syndromeJ Affect Disord 2020 266:429-46.10.1016/j.jad.2020.01.12432056910 [Google Scholar] [CrossRef] [PubMed]
[44]. Stanculete FM, Ismaiel A, Popa SL, Capatina OO, Irritable bowel syndrome and resilienceJ Clin Med 2023 12(13):422010.3390/jcm1213422037445254PMC10342810 [Google Scholar] [CrossRef] [PubMed]
[45]. Groeger D, Murphy EF, Tan HTT, Larsen IS, O’Neill I, Quigley EMM, Interactions between symptoms and psychological status in irritable bowel syndrome: An exploratory study of the impact of a probiotic combinationNeurogastroenterol Motil 2023 35(1):e1447710.1111/nmo.1447736178333PMC10078522 [Google Scholar] [CrossRef] [PubMed]
[46]. Doyle L, Cartwright T, Yoga use, physical and mental health, and quality of life in adults with irritable bowel syndrome: A mixed-methods studyEur J Integr Med 2023 62:10227010.1016/j.eujim.2023.102270 [Google Scholar] [CrossRef]
[47]. Goodoory VC, Mikocka-Walus A, Yiannakou Y, Houghton LA, Black CJ, Ford AC, Impact of psychological comorbidity on the prognosis of irritable bowel syndromeAm J Gastroenterol 2021 116(7):1485-94.10.14309/ajg.000000000000124733840729 [Google Scholar] [CrossRef] [PubMed]
[48]. Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, Groves D, Impairment in work productivity and health-related quality of life in patients with IBSAm J Manag Care 2005 11(1 Suppl):S17-S26. [Google Scholar]
[49]. Goodoory VC, Ng CE, Black CJ, Ford AC, Impact of Rome IV irritable bowel syndrome on work and activities of daily livingAliment Pharmacol Ther 2022 56(5):844-56.10.1111/apt.1713235794733PMC9543519 [Google Scholar] [CrossRef] [PubMed]
[50]. Paré P, Gray J, Lam S, Balshaw R, Khorasheh S, Barbeau M, Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: Baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic studyClin Ther 2006 28(10):1726-11.10.1016/j.clinthera.2006.10.01017157129 [Google Scholar] [CrossRef] [PubMed]
[51]. Stephenson JJ, Buono JL, Spalding WM, Cai Q, Tan H, Carson RT, Impact of irritable bowel syndrome with constipation on work productivity and daily activity among commercially insured patients in the United StatesValue Health 2014 17(7):A37010.1016/j.jval.2014.08.83927200787 [Google Scholar] [CrossRef] [PubMed]
[52]. Abel JL, Carson RT, Andrae DA, The impact of treatment with eluxadoline on health-related quality of life among adult patients with irritable bowel syndrome with diarrheaQual Life Res 2019 28(2):369-77.10.1007/s11136-018-2008-z30267294PMC6373309 [Google Scholar] [CrossRef] [PubMed]
[53]. Black CJ, Yiannakou Y, Guthrie EA, West R, Houghton LA, Ford AC, A novel method to classify and subgroup patients with IBS based on gastrointestinal symptoms and psychological profilesAm J Gastroenterol 2021 116(2):372-81.10.14309/ajg.000000000000097533110014 [Google Scholar] [CrossRef] [PubMed]
[54]. Quek SXZ, Loo EXL, Demutska A, Chua CE, Kew GS, Wong S, Impact of the coronavirus disease 2019 pandemic on irritable bowel syndromeJ Gastroenterol Hepatol 2021 36(8):2187-97.10.1111/jgh.1546633615534PMC8014795 [Google Scholar] [CrossRef] [PubMed]
[55]. Foxx-Orenstein AE, New and emerging therapies for the treatment of irritable bowel syndrome: An update for gastroenterologistsTherap Adv Gastroenterol 2016 9(3):354-75.10.1177/1756283X1663305027134665PMC4830102 [Google Scholar] [CrossRef] [PubMed]
[56]. Lacy BE, Weiser K, De Lee R, The treatment of irritable bowel syndromeTherap Adv Gastroenterol 2009 2(4):221-38.10.1177/1756283X091047942118054521180545 [Google Scholar] [CrossRef] [PubMed]
[57]. El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T, Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled studyGut 2020 69(5):859-67.10.1136/gutjnl-2019-31963031852769PMC7229896 [Google Scholar] [CrossRef] [PubMed]
[58]. Abdelghafar YA, AbdelQadir YH, Motawea KR, Nasr SA, Omran HAM, Belal MM, Efficacy and safety of fecal microbiota transplant in irritable bowel syndrome: An update based on meta-analysis of randomized control trialsHealth Sci Rep 2022 5(5):e81410.1002/hsr2.81436110348PMC9466358 [Google Scholar] [CrossRef] [PubMed]
[59]. Doll JPK, Vázquez-Castellanos JF, Schaub AC, Schweinfurth N, Kettelhack C, Schneider E, Fecal Microbiota Transplantation (FMT) as an adjunctive therapy for depression-case reportFront Psychiatry 2022 13:815422Published 2022 Feb 1710.3389/fpsyt.2022.81542235250668PMC8891755 [Google Scholar] [CrossRef] [PubMed]
[60]. Parker G, Spoelma MJ, Rhodes N, Faecal microbiota transplantation for bipolar disorder: A detailed case studyBipolar Disord 2022 24(5):559-63.10.1111/bdi.1318735165993PMC9545285 [Google Scholar] [CrossRef] [PubMed]