Case Report
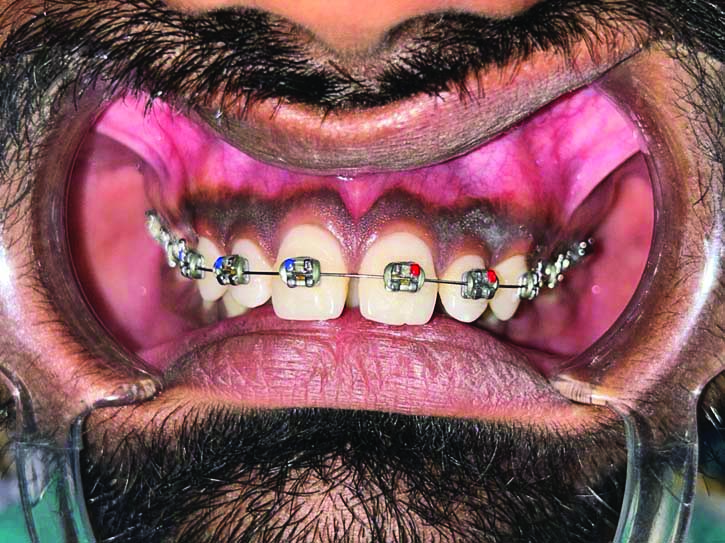
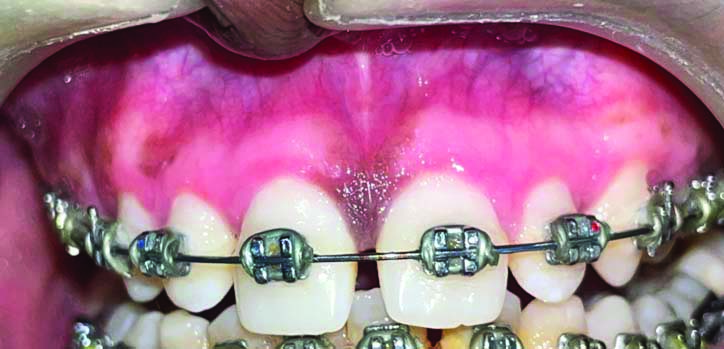
A 21-year-old-male patient reported to the Department of Periodontology, with the chief complaint of concern about dark colour of gums. His medical history suggested that he may have physiological melanin pigmentation, because this problem has existed since, his childhood. Melanin hyperpigmentation was found on both maxillary and mandibular labial gingiva [Table/Fig-1]. The patient was systemically healthy, had no harmful habits like smoking or tobacco chewing, and maintained good oral hygiene. The plaque index score was calculated using Turesky Modified Quigley-Hein Plaque Index and was found to be 1 [1]. Oral Hygiene Index Simplified (OHI-S) score [2] interpreted as good, with the score of 0.8. The dental history revealed the ongoing orthodontic treatment since, one month.
Showing preoperative photograph of hyperpigmented gingiva.
The patient was explained about all the treatment choices available, including the use of diodes, lasers, electrocautery, and the traditional scalpel procedure, along with their benefits and drawbacks. The possibility of repigmentation over time was also addressed. Patient found it comfortable proceed with conventional method of scalpel surgical technique for gingival depigmentation.
On the first visit, phase I therapy was conducted, which included scaling, polishing, and oral hygiene instructions. The Dummett Oral Pigmentation Index was recorded and calculation was found to be 2.75 [3]. The mandibular gingival area was not depigmented because the patient did not find it to be an aesthetic concern.
A split-mouth approach was planned to compare the conventional Coe-PakTM dressing and Blue®m oxygel as dressings after the surgical depigmentation. Blue®m oxygel has the combination of sodium percarbonate and honey enzymes, which are its active constituents, yields remarkable effects. Its unique quality is that it can release oxygen in the injured tissues at a therapeutic concentration [4].
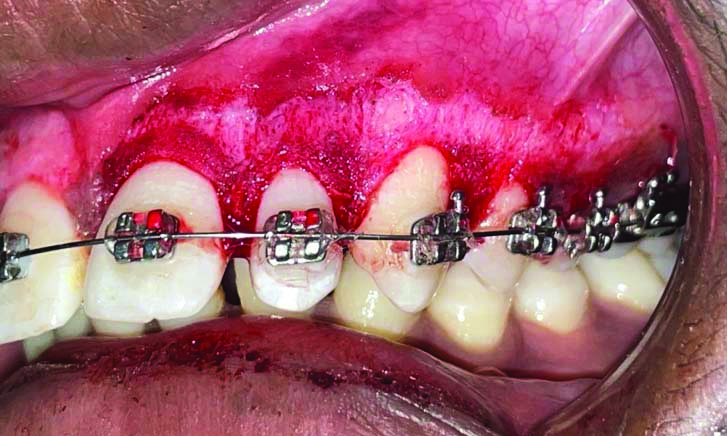
After taking the written informed consent, each half of the upper arch underwent the following steps: A 2% lignocaine was used for infiltration anaesthesia (Lignox 2% Indoco Warren), and using a no. 15 surgical blade, the entire pigmented layer of the gingival epithelium and some of the underlying connective tissue were removed [Table/Fig-2]. Pigmentation was observed extending from base of the interdental papilla to the mucogingival junction. Sterile gauze was employed to apply direct pressure and manage bleeding throughout the procedure.
Showing depigmentation of gingiva done using surgical scalpel technique.
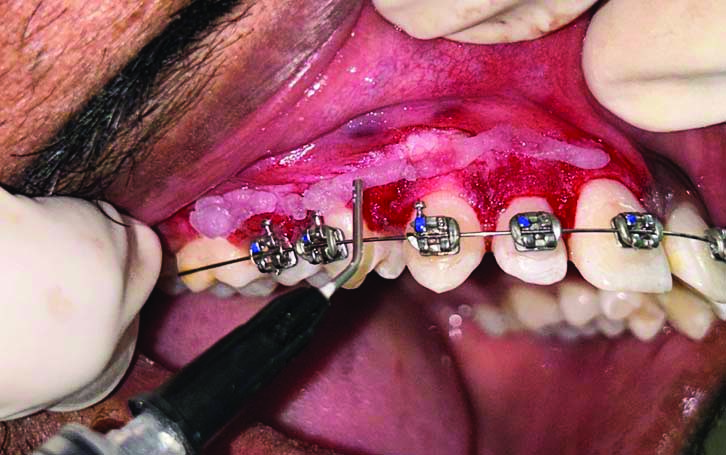
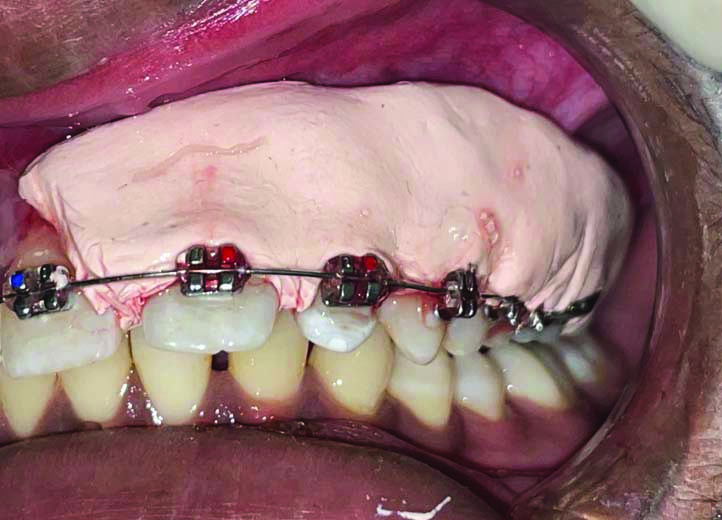
The depigmented area in the right upper quadrant was covered with Blue®m Gel [Table/Fig-3,4], while the left upper quadrant received the conventional Coe-PakTM periodontal dressing [Table/Fig-5].
Showing placement of Blue®m gel on right upper quadrant after surgical depigmentation.
Commercially available Blue®m gel and syringe used for application.

Showing placement of Coe-PakTM periodontal dressing on left upper quadrant after surgical depigmentation.
The patient was provided with five packets of Blue®m gel and instructed to apply it to the right quadrant at night after drying the site with a cotton pellet. Postoperative instructions were given, and the patient was prescribed non-steroidal anti-inflammatory medication, aceclofenac 200 mg, to be taken twice daily for three days if needed. The patient was called for follow-up after one week, one month, and three months for re-evaluation [Table/Fig-6,7,8,9 and 10].
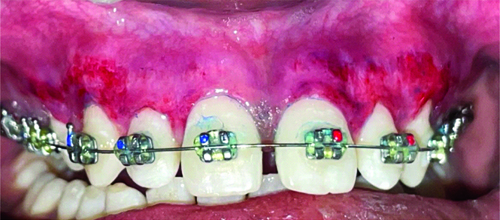
Showing follow-up after one week.
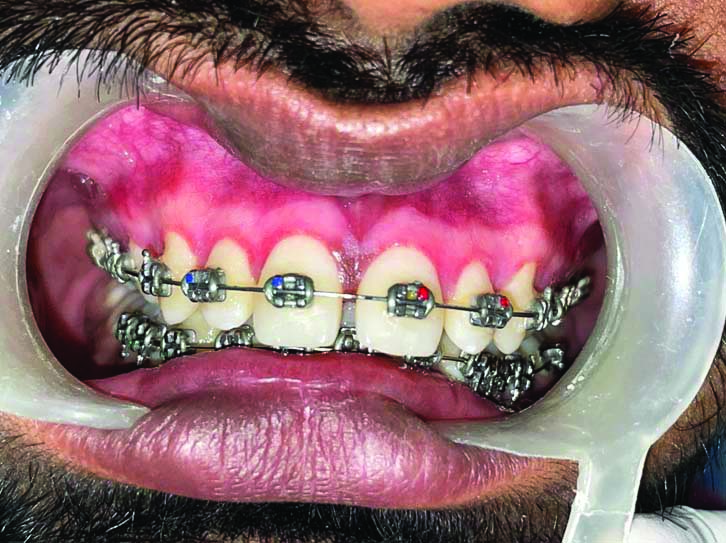
Showing follow-up after one month (right-side Blue M gel).
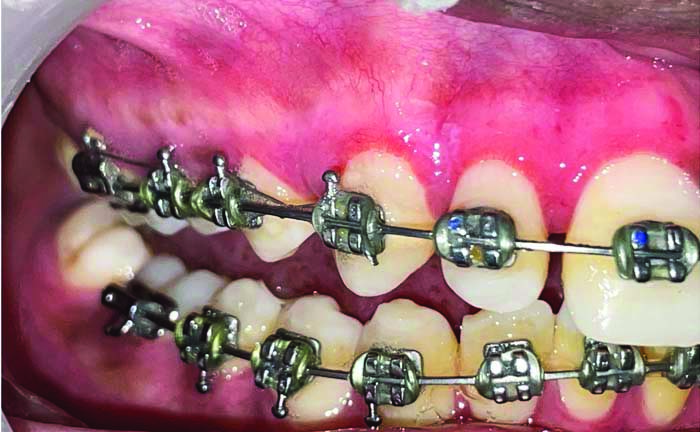
Showing follow-up after one month (Left-side Coe-PakTM).
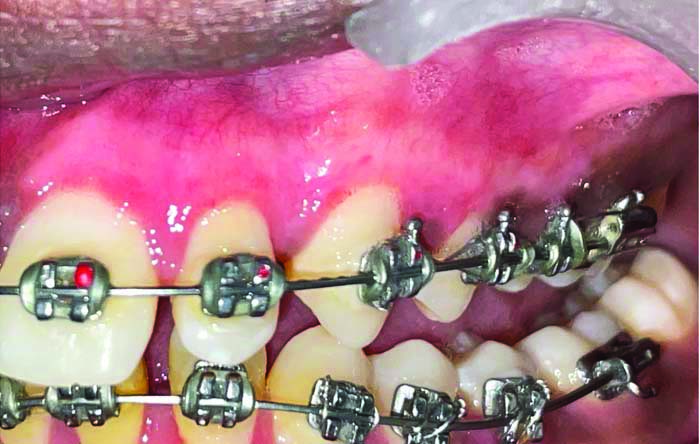
Showing healing at one month (frontal view).
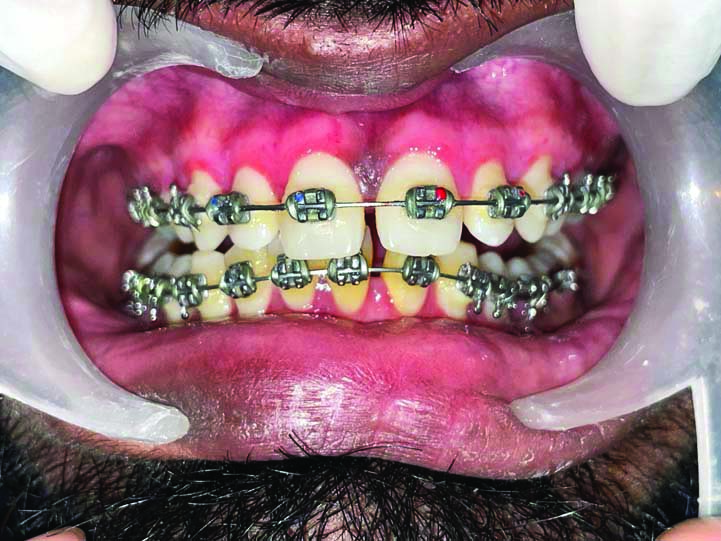
Showing healing at three months (frontal view).
The pack was removed after one week using dental tweezers and a blunt probe, and the surgical site area was investigated. The surgical case was requested to complete an assessment questionnaire consisting of 10 questions, rating their choice of dressing from 0 to 10 based on pain perception at different time intervals, comfort, taste, appearance, retention of the pack, sensation of burning, bleeding, swelling, and sensitivity experienced with each type of dressing. Each question was graded on a 0 to 10 Visual Analogue Scale (VAS) score:
0=no pain,
1-3=mild pain,
4-6=moderate pain,
7-10=severe pain [5].
The average score from all the 10 question responses was calculated at one week, one month, and three months.
On collecting the data, it was inferred that use of Blue®m gel was more acceptable, less painful, and as minimal discomfort compared to conventional dressing. Wound healing was uneventful on both areas. The one-week, one-month, and three-months postoperative follow-up, re-epithelisation was checked with the help of acetic acid and toluidine blue [Table/Fig-11], and Dummett’s Oral Pigmentation Index was calculated for both sides, with the scoring criteria being [3]:
Evaluation of re-epithelisation with the help of acetic acid and toluidine blue.
1: No clinical pigmentation;
2: Mild clinical pigmentation;
3: Moderate clinical pigmentation;
4: Heavy clinical pigmentation.
It was found that re-epithelisation was more with Blue®m gel than with Coe-PakTM.
During the initial and subsequent visits at one week, one month, and three months, there were no incidents of postoperatively, bleeding, sepsis, or scarring at any of the sites. Healing proceeded smoothly without any hitch. The gingival colour was found to be pink, in colour healthy in appearance, and firm in consistency, resulting in an a usual look. The patient was satisfied with the procedure, and the results were deemed excellent. After a three-month follow-up, mild repigmentation was observed only at particular sites [Table/Fig-12].
Showing re-epithelialisation at different follow-up periods.
| Variables | One week | One month | Three months |
|---|
| VAS | Re-epithelisation | VAS | Re-epithelisation | VAS | Re-epithelisation |
|---|
| Blue M gel | 2 | 0 | 1 | 1 | 0 | 2 |
| Coe-PakTM | 4 | 0 | 3 | 1 | 1 | 3 |
Discussion
A smile’s overall appearance and facial attractiveness are greatly influenced by gingival colour [6]. The gingiva is the most prevalent location of mucosal pigmentation, and the most frequent biological pigment is melanin, which is responsible for intrinsic discolouration of the gums [7]. Melanin pigmentation occurs to be a physiological process that is present across all races and is not indicative of any danger [8]. The extent of melanin pigmentation varies among individuals, primarily due to differences in the activity of melanoblasts. The typical gingival structure is unaffected by the symmetrical, continuous gingival melanin pigmentation. This condition is commonly observed in dark-skinned ethnic groups and can also occur in medically associated conditions such as Addison’s syndrome, Peutz-Jegher’s syndrome, and Von Recklinghausen’s disease (neurofibromatosis) [9].
Gingivectomy, mucosal excision using a scalpel, abrasion procedures, free gingival grafts, chemical methods utilising caustic chemicals, electrosurgery, cryotherapy, and the use of newly created lasers are some of the treatment modalities that have been employed to obtain pigmentation-free gums [10].
Among all the known methods for gingival depigmentation, surgical scalpel technique remains the gold standard due to its advantage of being cost-effective, better wound healing properties, and lesser chances of recurrence. Several disadvantages were included but among them the most frequently faced were excessive bleeding and healing with secondary intentions which made it compulsory to apply dressing to the surgical site. The most traditional and widely accepted procedure for gingival depigmentation is the scalpel technique, which involves removing a layer of connective tissue along with the gingival epithelium. This exposed connective tissue heals rather by secondary intention, resulting in melanin-free epithelium [11]. While this method promotes faster wound healing, it tends to be more painful. Since, secondary intentional healing takes place for the exposed tissues, a periodontal dressing is required to decrease bleeding, ache, and the risk of surgical site infection [12].
Careful attention is needed to safeguard the marginal gingiva during this procedure, as improper use can cause gingival recession and damage to the periosteum and bone [11].
Various periodontal dressing are available in three forms: periodontal dressings containing zinc oxide and eugenol, like Ward’s Wondrpak and periodontal dressing containing zinc oxide without eugenol like CoePak and PeriPac; and periodontal dressing without zinc oxide or eugenol [13]. The conventional Coe-PakTM dressing is the most popularly used periodontal dressing. It is packaged in two tubes, which are mixed right before usage. Zinc oxide, oil, gum, and lorothidol are contained in one tube, while liquid coconut fatty acids thickened with colophony resin and chlorothymol are contained in the other [14]. It is known for its strong adhesive properties and includes bacteriostatic agents. Unlike some periodontal dressings, it does not contain tissue stimulants like eugenol. Despite its widespread use, it has several drawbacks, including poor appearance, an unclear setting time, and challenges with flow characteristics during preparation. Its bulky and delicate nature has also posed ongoing issues [15].
Recently, Blue®m gel was developed with an active oxygen composition to address this problem. The combination of this gel’s active components, honey enzymes, and sodium percarbonate has produced amazing results. Oxygen is released into affected tissues at therapeutic concentrations [13]. Blue®m gel shows action against bacteria, histamine, and has mycotic properties, in addition to encouraging neovascularisation, eliminating toxins, triggering the synthesis of new blood cells, and boosting stem cell development for quicker healing [16]. Given the advantages of gradual oxygen release for improving wound healing and the positive results of a few clinical trials, the case was conducted to compare and evaluate the efficacy of the popular periodontal dressing with that of recent commercially available gel. The overall outlook is quite favourable, but long-term follow-up is required.
In a study by Juliana H and Tarek S, the effects of the conventional Coe-PakTM dressing and reactive oxygen species gel (Blue®m gel) on gingival healing and discomfort after surgical depigmentation were compared [17]. A total of 20 patients participated in the study. Statistically significant differences in pain were observed after 1, 2, 3, 4, and 5 days, with the Blue®m gel group showing a marked improvement. Additionally, the Blue®m gel group demonstrated a significantly higher re-epithelisation index after one, two, and three weeks. Thus, Blue®m gel emerges as a promising alternative to Coe-PakTM dressing, offering better pain reduction, accelerated wound healing, and enhanced postoperative re-epithelisation [18].
Another similar study by Garg U et al., aimed to assess and contrast the clinically the effects of a standard periodontal dressing (Coe-PakTM) and an oxygenating agent (Blue®m gel) on patient preference measures, such as discomfort and wound healing, after surgical depigmentation [18]. The results demonstrated that the pain and re-epithelisation scores were significantly lower in the Blue®m gel group compared to the Coe-PakTM group. Thus, Blue®m gel can be considered a better alternative to Coe-PakTM as a periodontal dressing due to its superior healing properties and ability to reduce postoperative pain [18].
Conclusion(s)
Based on the results from this clinical case, it was concluded that the use of Blue®m gel following surgical scalpel technique for depigmentation enhances wound healing, accelerates re-epithelisation, and reduces postoperative pain more effectively than applying the traditional Coe-PakTM dressing. However, further more extensive and long-term follow-up studies are required.
[1]. Turesky S, Gilmore ND, Glickman I, Reduced plaque formation by the chloromethyl analogue of victamine CJ Periodontol 1970 41(1):41-43.10.1902/jop.1970.41.1.415264376 [Google Scholar] [CrossRef] [PubMed]
[2]. Greene JC, Vermillion JR, The simplified oral hygiene indexJ Am Dent Assoc 1964 68:07-13.10.14219/jada.archive.1964.003414076341 [Google Scholar] [CrossRef] [PubMed]
[3]. Dummett CO, Physiologic pigmentation of the oral and cutaneous tissues in the negroJ Dent Res 1946 25(6):421-32.10.1177/0022034546025006020120285102 [Google Scholar] [CrossRef] [PubMed]
[4]. A unique formula that makes us different - blue®m [Internet][cited 2024 Oct 5]. Available from: https://bluemcare.com/stories/unique-formula-makes-us-different/?srsltid=AfmBOoqu8Skb-8Lxn7WDRoUuQHQEZQLFgjyIt7_qRUQzbAwx9CK4wp1B [Google Scholar]
[5]. Delgado DA, Lambert BS, Boutris N, McCulloch PC, Robbins AB, Moreno MR, Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adultsJ Am Acad Orthop Surg Glob Res Rev 2018 2(3):e08810.5435/JAAOSGlobal-D-17-0008830211382PMC6132313 [Google Scholar] [CrossRef] [PubMed]
[6]. Pham TAV, Nguyen PA, Morphological features of smile attractiveness and related factors influence perception and gingival aesthetic parametersInt Dent J 2022 72(1):67-75.10.1016/j.identj.2021.02.00133707026PMC9275111 [Google Scholar] [CrossRef] [PubMed]
[7]. Disorders of oral pigmentation: Background, pathophysiology, etiology 2021 [Cited 2024 Oct 5]; Available from: https://emedicine.medscape.com/article/1078143-overview?form=fpf [Google Scholar]
[8]. Thawabteh AM, Jibreen A, Karaman D, Thawabteh A, Karaman R, Skin Pigmentation types, causes and treatment—A reviewMolecules 2023 28(12):483910.3390/molecules2812483937375394PMC10304091 [Google Scholar] [CrossRef] [PubMed]
[9]. McGarrity TJ, Amos CI, Baker MJ, Peutz-Jeghers Syndrome. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, et al., editorsGeneReviews® [Internet] 1993 Seattle [WA]University of Washington, Seattle[cited 2024 Jun 10]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1266/ [Google Scholar]
[10]. Patil K, Joshi V, Waghmode V, Kanakdande V, Gingival depigmentation: A split mouth comparative study between scalpel and cryosurgeryContemp Clin Dent 2015 6:97-101.10.4103/0976-237X.15296425821386PMC4374330 [Google Scholar] [CrossRef] [PubMed]
[11]. Roshna T, Nandakumar K, Anterior esthetic gingival depigmentation and crown lengthening: Report of a caseJ Contemp Dent Pract 2005 6(3):139-47.10.5005/jcdp-6-3-13916127483 [Google Scholar] [CrossRef] [PubMed]
[12]. Grover HS, Dadlani H, Bhardwaj A, Yadav A, Lal S, Evaluation of patient response and recurrence of pigmentation following gingival depigmentation using laser and scalpel technique: A clinical studyJ Indian Soc Periodontol 2014 18(5):586-92.10.4103/0972-124X.14245025425820PMC4239748 [Google Scholar] [CrossRef] [PubMed]
[13]. Baghani Z, Kadkhodazadeh M, Periodontal dressing: A review articleJ Dent Res Dent Clin Dent Prospects 2013 7(4):183-91. [Google Scholar]
[14]. Pera C, Ueda P, Casarin RCV, Ribeiro FV, Pimentel SP, Casati MZ, Double-masked randomized clinical trial evaluating the effect of a triclosan/copolymer dentifrice on periodontal healing after one-stage full-mouth debridementJ Periodontol 2012 83(7):909-16.10.1902/jop.2011.11034822087803 [Google Scholar] [CrossRef] [PubMed]
[15]. Sharmila V, Saichandran S, Babu TA, Singh D, A rare case of bilateral ovarian fibroma presenting as Meigs syndromeJ Obstet Gynaecol 2013 33(6):636-37.10.3109/01443615.2013.79912823919874 [Google Scholar] [CrossRef] [PubMed]
[16]. Rodriguez PG, Felix FN, Woodley DT, Shim EK, The role of oxygen in wound healing: a review of the literatureDermatol Surg 2008 34(9):1159-69.10.1097/00042728-200809000-0000118513296 [Google Scholar] [CrossRef] [PubMed]
[17]. Juliana H, Tarek S, Comparative study of the effect of BlueM active oxygen gel and coe-pack dressing on postoperative surgical depigmentation healingThe Saudi Dent J 2022 34(4):32810.1016/j.sdentj.2022.04.00535692245PMC9177883 [Google Scholar] [CrossRef] [PubMed]
[18]. Garg U, Kaur N, Kaur G, Comparative evaluation of oxygenating agent “blue m gel” and traditional periodontal dressing “Coe-Pak” on patient preference parameters (Pain and wound healing) after surgical depigmentation- A split mouth studyIP Int J Periodontol Implantol 2024 9(1):31-37.10.18231/j.ijpi.2024.008 [Google Scholar] [CrossRef]