Myocardial viability refers to a phenomenon in which dysfunctional myocardium, due to acute or chronic ischaemia, retains the potential to recover its systolic function after revascularisation. The myocardium sustains irreparable damage as a result of cardiac insults resulting from acute or chronic ischaemia, but it can also adapt by remaining in a latent condition in the ischaemic environment, with the possibility of recovery upon revascularisation [1].
Extracellular volume expansion and myocyte death are two of the basic histologic alterations in necrotic myocardium. A viability investigation using DE-CMR is made possible by this alteration, which permits the high absorption and retention of high-density gadolinium-based contrast agents in the cell, as well as, extracellular space [2].
The link between hyper-enhanced myocardium during CE-MRI and myocardial necrosis evaluated by histology has been studied in various experimental trials [3-8]. Areas of irreversible injury identified by tetrazolium staining were found to be identical in size and shape to the hyper-enhanced areas at CE-MRI using high spatial resolution techniques [9]. Additionally, depending on the transmural extent of hyper-enhancement, CE-MRI enables one to distinguish between regions with reversible and irreversible myocardial damage [8,9]. Furthermore, in experimental studies [10] and in patients with acute MI and chronic ischaemic heart disease, the transmural extent of hyper-enhancement has been found to be predictive of recovery of segmental contractile function after myocardial revascularisation [11,12].
Delayed Enhancement Magnetic Resonance Imaging (DE-MRI) can identify irreversibly injured myocardium and identify regions where functional recovery following revascularisation is unlikely [13]. A study using cardiac MRI was a relatively newer and non-invasive approach in the host institute and it helped provide an insight into the tremendous repercussions of the absence of such an imaging technique in the proper management of the affected individuals. Hence, the study was aimed at building a wider acceptance of the utility of cardiac MRI in ischaemic heart disease affected patients and its fecund ability in helping to categorise and provide the better management path according to the extent of damage which was elicited using the purported study methodology.
Materials and Methods
The present hospital-based prospective observational study was conducted in the Department of Radiodiagnosis, Gauhati Medical College and Hospital, Guwahati, Assam, India, from April 2021 to September 2022. Before starting the study, Institutional Ethical Committee (IEC) clearance was obtained (MC/190/2007/Pt-II/July 2021/TH-52). A total of 50 patients were included in the study by convenient sampling.
Inclusion criteria: Patients diagnosed with Chronic Ischaemic Heart Disease (with a prior history of MI) based on prior Electrocardiogram (ECG) and Echocardiography (Echo) findings were included in the study.
Exclusion criteria: Patients with high creatinine levels (>1.2 mg/dL) were excluded from the study.
Study Procedure
Imaging technique: MRI evaluations were performed on 3 Tesla Siemens Magnetom Skyra machine. MRI was performed on supine patients with optimal placement and body stabilisation. Standardised retrograde ECG gating was done. A standard body array 18 channel 3 Tesla (3T) Tim coil was used.
To localise the heart for anatomy and position, localiser sequences were first performed in a variety of planes. Precontrast scanning was carried out for both function and morphology. In order to evaluate cardiac function, two-chamber Cine True Fast Imaging with Steady-state Free Precession (TRUFI) in long axis, four-chamber CINE TRUFI in long axis, CINE TRUFI retro in short axis, Cine Left Ventricular Outflow Tract (LVOT) and Cine Right Ventricular Outflow Tract (RVOT) MRI sequences were employed. The sequences employed for morphology include T2 TRUFI, T2 Half-Fourier-Acquired Single-shot Turbo Spin Echo (HASTE) dark blood, T2 TSE dark blood, T1 TSE dark blood, and T2 Short Tau Inversion Recovery (STIR) dark blood.
Perfusion and viability investigations were conducted using contrast media containing gadobutrol 1.0 mmol/mL (0.1 mmol/kg body weight). The authors employed the 50-phase DYNAMIC TRUFI SR Extracorporeal Pulse Activation Therapy (EPAT) sequence for perfusion, using a 2-chamber, 4-chamber and short axis view for the left side of the heart and a 2-chamber view for the right side. Following viability imaging, Inversion Time (TI) scout was taken in the short axis after approximately 10 minutes of contrast injection to assess the TI value. This TI value was further used for the delayed TRUFI high-resolution Phase-sensitive Inversion Recovery (PSIR) sequence. The MRI images were analysed to assess cardiac morphology, function, perfusion and myocardial viability.
Imaging evaluation: Seventeen-segment cardiac model, as described by the American Heart Association (ASA) [14] was used in the description of the perfusion defects, wall motion abnormality and extent of LGE [Table/Fig-1,2]. An area of ischaemic myocardium showed up as a regional wall motion abnormality in the cine MR images and perfusion defect in perfusion imaging. The infarcted tissues showed up as segmental hyperenhancement of variable transmurality on the post-gadolinium-Diethylenetriamine Pentaacetic Acid (DTPA) delayed scans.
A 17-segment model was used for analysis.
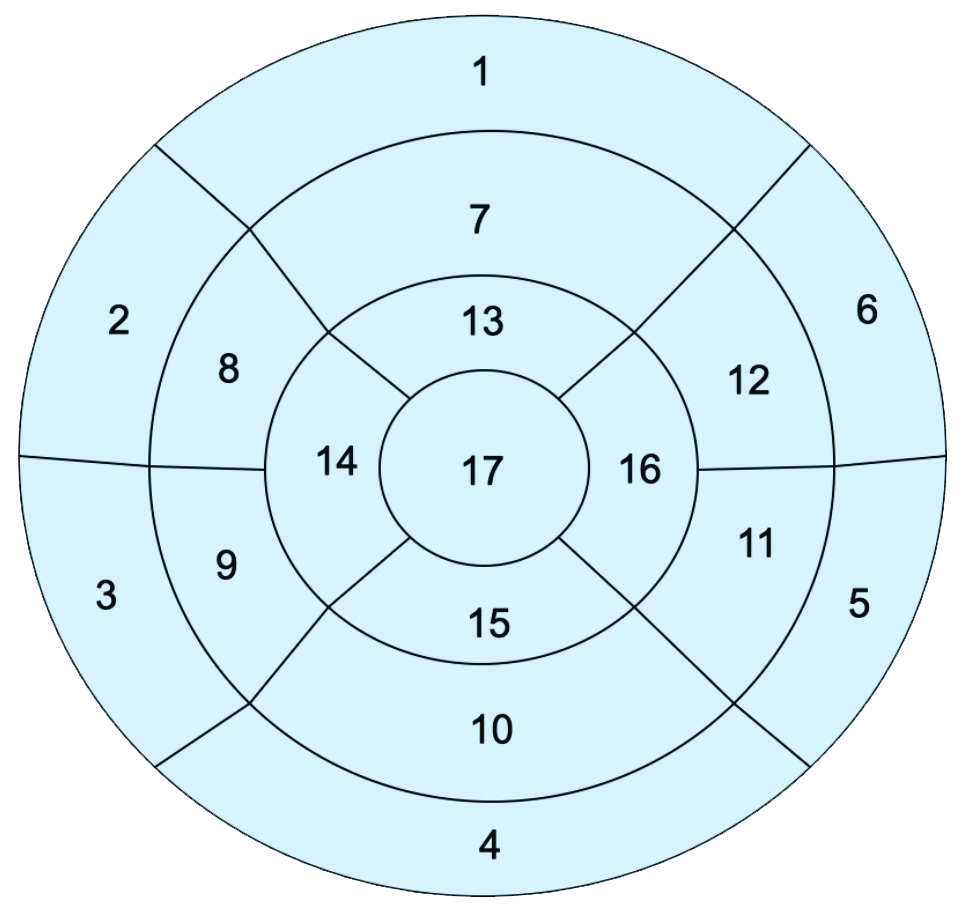
| Basal segments | Mid-cavity segments | Apical segments |
|---|
| 1. Basal anterior | 7 Mid anterior | 13 Apical anterior |
| 2. Basal anteroseptal | 8 Mid anteroseptal | 14 Apical septal |
| 3. Basal inferoseptal | 9 Mid inferoseptal | 15 Apical inferior |
| 4. Basal inferior | 10 Mid inferior | 16 Apical lateral |
| 5. Basal inferolateral | 11 Mid inferolateral | 17 Apex |
| 6. Basal anterolateral | 12 Mid anterolateral | |
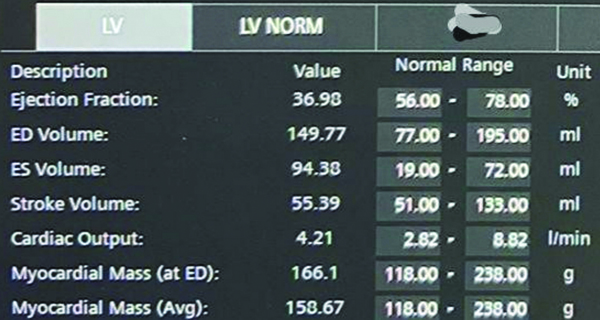
Left ventricular function: The LV ejection fraction was calculated by the Siemens 3T MRI machine-specific software, which is a dedicated software provided by the same company. The software used is Numaris syngo software version 4 (Siemens) (Syngo MR E11). The software allows us to outline the endocardial and epicardial contours in multiple planes of short axis views of both the ventricles at end diastole and end systole. The machine then generates the functional parameters from this [Table/Fig-3,4].
Endocardium and epicardium contours in short axis view of the left ventricle at mid cavity at end diastole, using Syngo workstation.
Left ventricular function generated by Syngo workstation.
LV thickening: LV Wall-thickness (LVWT) was measured in an end-diastolic cine image [Table/Fig-5,6] [15].
LV end-diastolic wall thickness measurement at base, using Syngo workstation.
LV end-diastolic myocardial thickness grading [15].
| LV end-diastolic myocardial wall thickness | Grading |
|---|
| <11 mm | Normal |
| 11-13 mm | Mild |
| 14-15 mm | Moderate |
| >15 mm | Severe |
Wall motion abnormality was scored qualitatively by visual eyeballing method and scored as: 0- normal, 1- mild or moderate hypokinesia, 2- severe hypokinesia, 3- akinesia, and 4- dyskinesia.
Perfusion imaging: Myocardial perfusion was done by monitoring the “first pass” of contrast material through the heart with a bolus gadolinium injection in conjunction with ultrafast cine MR sequences. Perfusion defect was assessed by the presence of segmental myocardial hypoenhancement, that reflects a relative hypoperfusion in comparison to other territories.
LGE scoring: The Inversion Time (TI) at which the myocardium is nulled was chosen for use in delayed enhancement imaging. This image showed the appearance of normal nulled myocardium using an Inversion Recovery (IR) pulse sequence. The normal nulled myocardium had no signal and appeared dark. So a pathological myocardium which showed enhancement was better visualised when signal from surrounding normal myocardium was nulled. The T1 time at which whole myocardium is nulled is called T1 null time or T1 inversion time. It was taken as 10 minutes in our study based on previous literatures. Each myocardial sector was then evaluated for the presence of hyperenhancement, defined as an area of signal enhancement ≥3 SD of the signal of non enhanced myocardium, based on previous studies done elsewhere. The total myocardial area and contrast-enhanced area per sector are traced manually. The extent of contrast enhancement is expressed as a percentage of the total myocardial area (A_hyperenhanced/A_myocardium×100, where A denotes area) [Table/Fig-7] [16]. A 50% transmurality of LGE was taken as a cut-off to stratify patients into two groups and to guide further course of treatment based on previous literature [17]. Seventeen patients had LGE ≤50% and 33 patients had LGE >50%. Patients with ≤50% transmurality of LGE were taken up for coronary revascularisation. Of patients with >50% transmurality, 15 patients were given conservative treatment, and 18 patients were taken up for coronary revascularisation (patients who had better chances of recovery based on clinical, ECG and Echo findings were taken up for surgery). The patients were then followed-up at 6-month interval to know the status of the patient after treatment, by clinical examination and Echo. Recovery of the patient was evaluated based on the following criteria:
Improvement in clinical symptoms;
Improvement of ejection fraction on Echo;
No further attacks of MI.
Late Gadolinium Enhancement (LGE) scoring [16].
| Score | Enhancement percentage |
|---|
| 0 | No enhancement |
| 1 | 0-25% |
| 2 | 26-50% |
| 3 | 51-75% |
| 4 | 76-100% |
Statistical Analysis
Data were entered in Microsoft Excel sheet and analysed using Statistical Package for the Social Sciences (SPSS) software for Windows, version 26.0. The quantitative values, like age and LVEF, were summarised as mean (SD) based on distribution of data. Pearson’s correlation test was done to check the correlation between various parameters. The p-value was calculated using the Chi-square test, and a p-value of less than 0.05 was considered statistically significant.
Results
A total number of 850 segments were studied. Out of the 50 study subjects, the youngest patient was 31 years old, while the oldest was 78 years old. The mean age was 52±14 years. A total of 27 (54%) patients were males and 23 (46%) patients were females.
A total of 41 (82%) patients showed dyslipidemia, 26 (52%) patients were obese, 27 (54%) patients had sedentary lifestyle, and 25 (50%) patients lead a stressful life [Table/Fig-8].
Distribution of co-morbidities in patients.
| Co-morbidities | LGE ≤50% | LGE >50% | Total | p-value(Chi-square test) |
|---|
| n | Percentage (%) | n | Percentage (%) | n | Percentage (%) |
|---|
| DM | No | 4 | 23.5 | 9 | 27.3 | 13 | 26 | 0.024 |
| Yes | 13 | 76.5 | 24 | 72.7 | 37 | 74 |
| HTN | No | 3 | 17.6 | 9 | 27.3 | 12 | 24 | 0.003 |
| Yes | 14 | 82.4 | 24 | 72.7 | 38 | 76 |
| Obesity | No | 7 | 41.2 | 17 | 51.5 | 24 | 48 | 0.042 |
| Yes | 10 | 58.8 | 16 | 48.5 | 26 | 52 |
| Dyslipidemia | No | 3 | 17.6 | 6 | 18.2 | 9 | 18 | 0.013 |
| Yes | 14 | 82.4 | 27 | 81.8 | 41 | 82 |
| Sedentery lifestyle | No | 7 | 41.2 | 16 | 48.5 | 23 | 46 | 0.023 |
| Yes | 10 | 58.8 | 17 | 51.5 | 27 | 54 |
| Stress | No | 12 | 70.6 | 13 | 39.4 | 25 | 50 | 0.037 |
| Yes | 5 | 29.4 | 20 | 60.6 | 25 | 50 |
| Smoking | No | 4 | 23.5 | 7 | 21.2 | 11 | 22 | 0.017 |
DM: Diabetes mellitus; HTN: Hypertension
Out of 50 patients, ECG could detect an infarct in 40 (80%) patients, and could not detect an infarct in the remaining 10 (20%) patients. However, cardiac MRI could detect an infarct in all patients. Hence, cardiac MRI was found to be superior in detecting an infarct compared to ECG (p-value<0.05).
The mean ejection fraction detected by Echo was 39.4±5% and the mean ejection fraction detected by cardiac MRI was 39.6±5%. There was a positive correlation between the two (p-value=0.0001) (Pearson’s correlation=0.952) [Table/Fig-9].
Correlation between LVEF detected by Echo and by cardiac MRI.
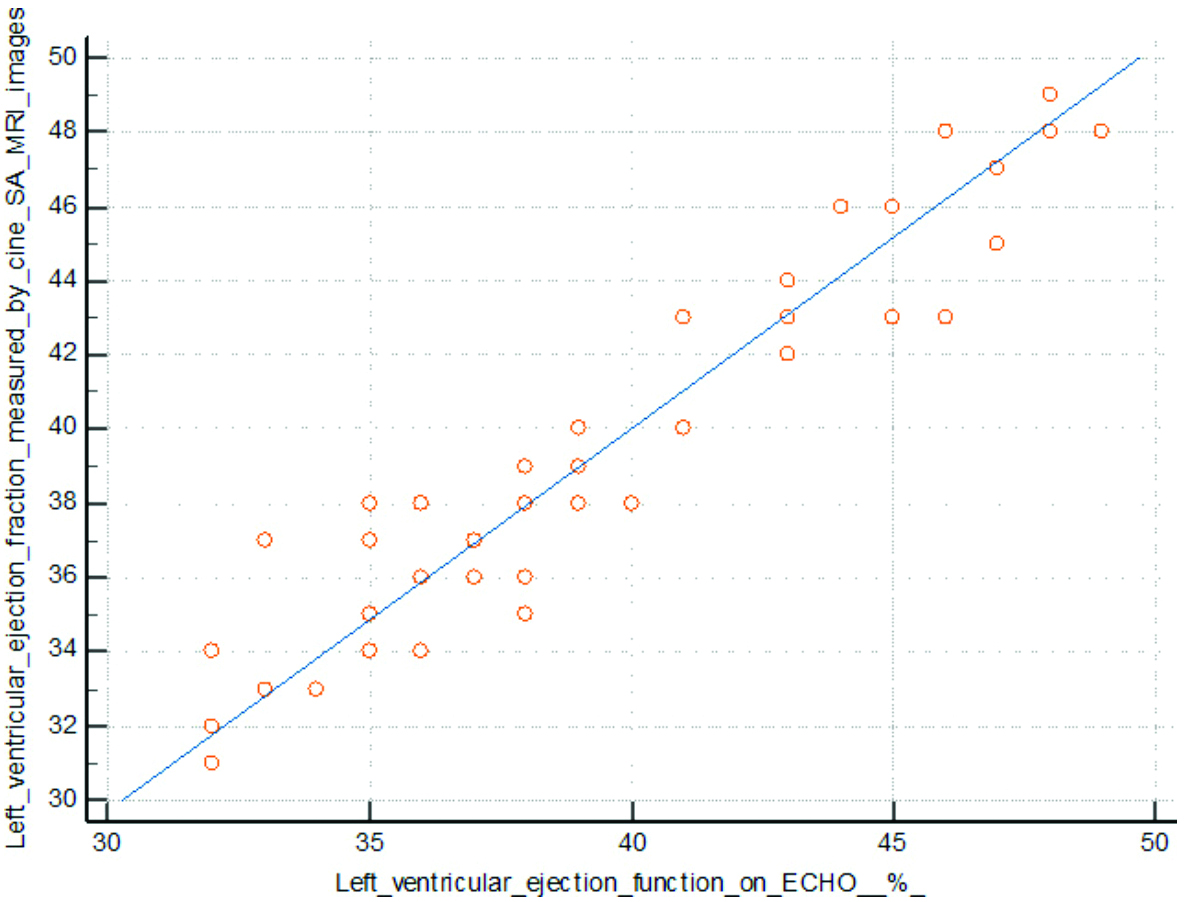
The mean ejection fraction of patients with LGE score ≤50% (45±2%) was higher than the mean ejection fraction of patients with LGE score >50% (36±5%), which was found to be statistically significant (p-value=0.001).
Out of 850 segments, 564 segments showed wall motion abnormality detected by Echo, while 606 segments showed wall motion abnormality detected by cardiac MRI. There was a positive correlation between wall motion abnormality identified by Echo and that identified by cardiac MRI (p-value=0.0001) (Pearson’s correlation=0.974) [Table/Fig-10].
Correlation between wall motion abnormality detected by Echo and cardiac MRI.
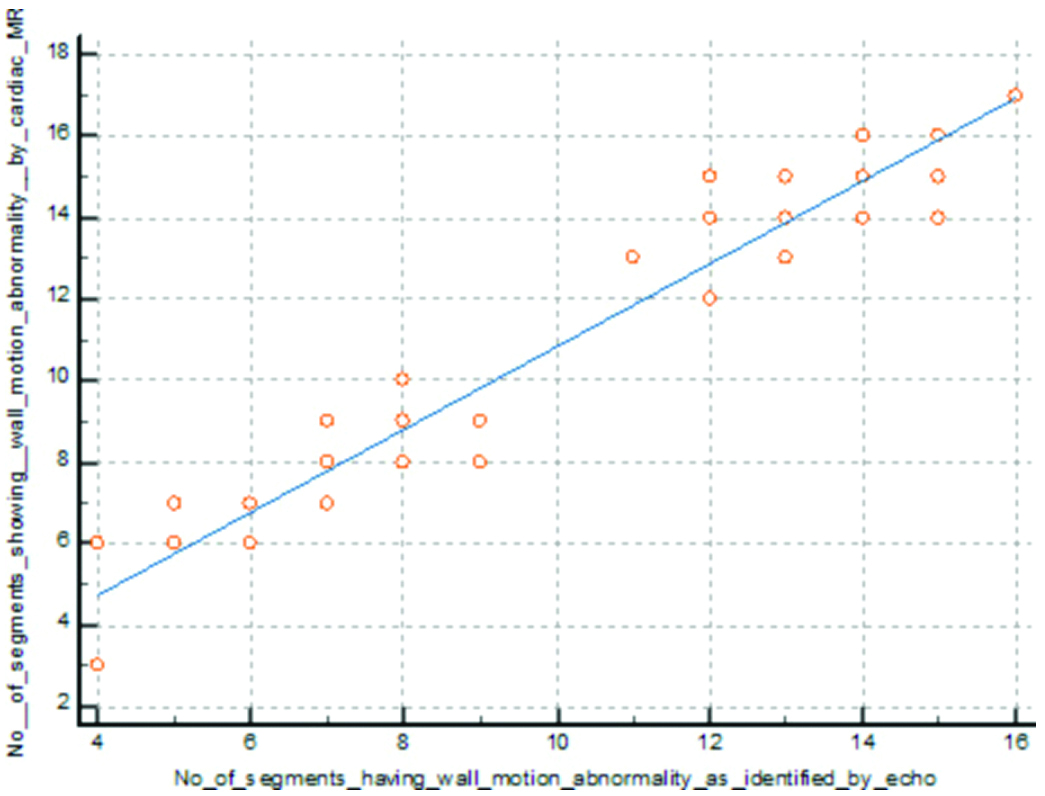
Out of 850 segments, 606 segments showed regional wall motion abnormality detected by cardiac MRI, and 307 segments showed LGE. A positive correlation seen between regional wall motion abnormality detected by cardiac MRI and LGE (p-value=0.0001) (Pearson’s correlation=0.886) [Table/Fig-11].
Correlation between wall motion abnormality and delayed enhancement in cardiac MRI.
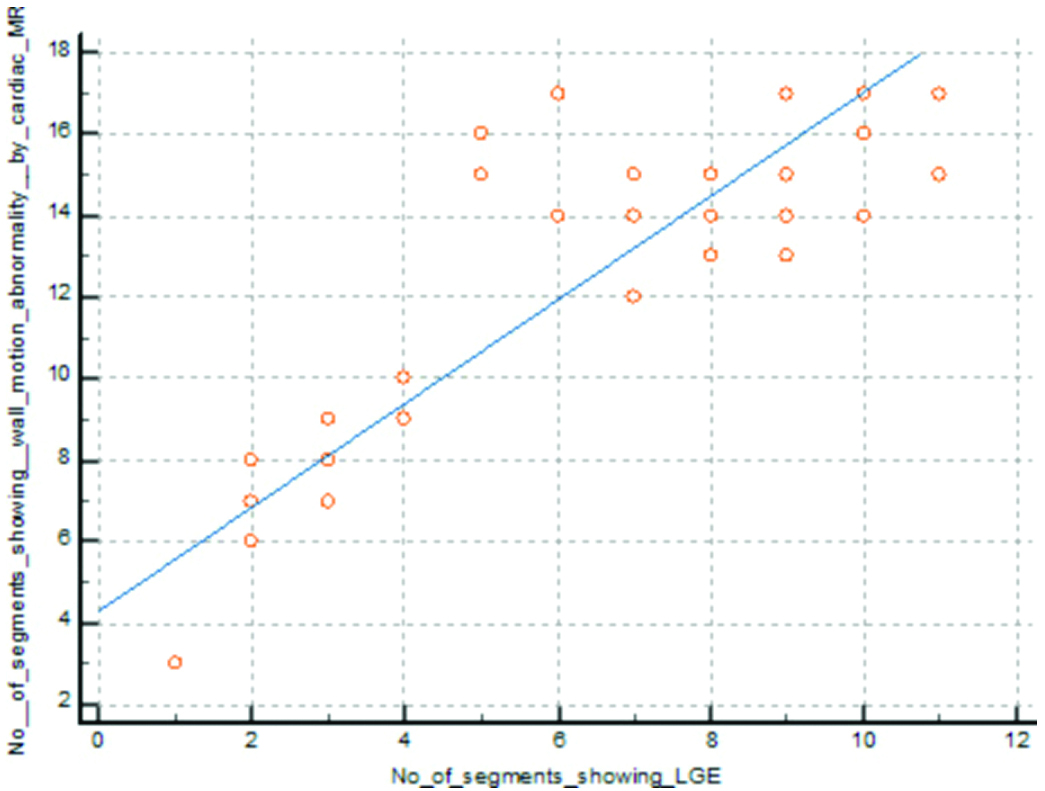
Out of 850 segments, 606 segments showed wall motion abnormality, and 380 segments showed perfusion defects detected by cardiac MRI. There was a positive correlation between wall motion abnormality and perfusion defect detected by cardiac MRI (p-value=0.0001) (Pearson’s correlation=0.954) [Table/Fig-12].
Correlation between wall motion abnormality and perfusion defect detected by cardiac MRI.
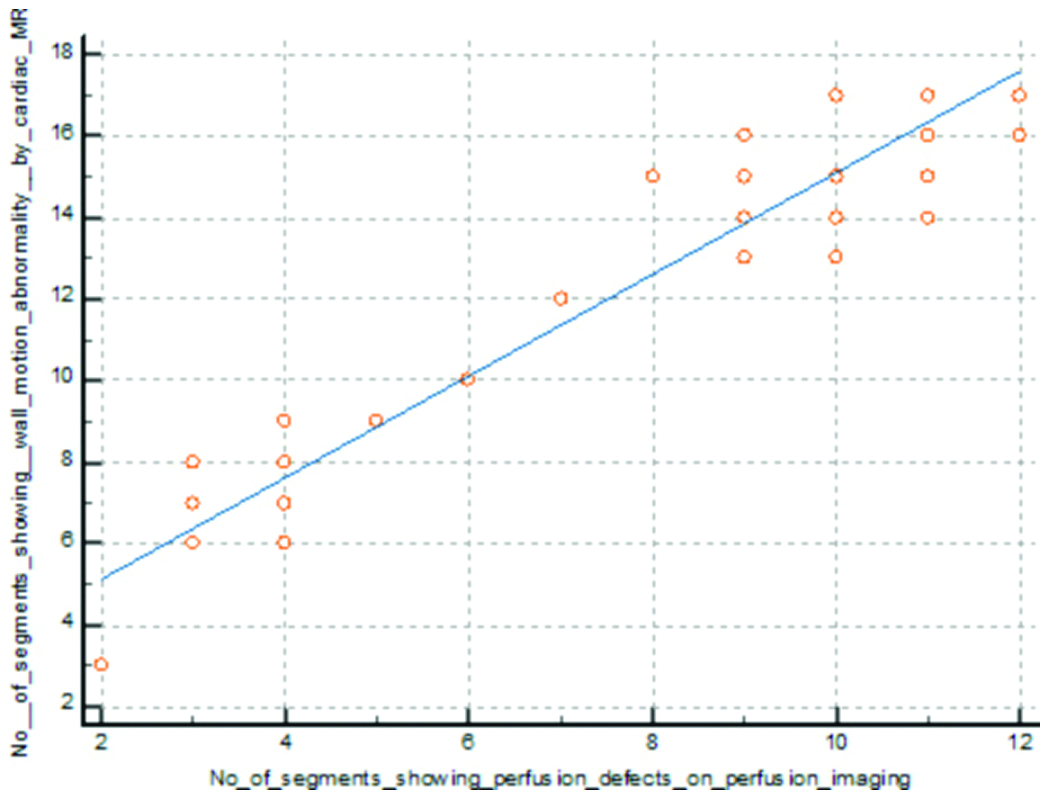
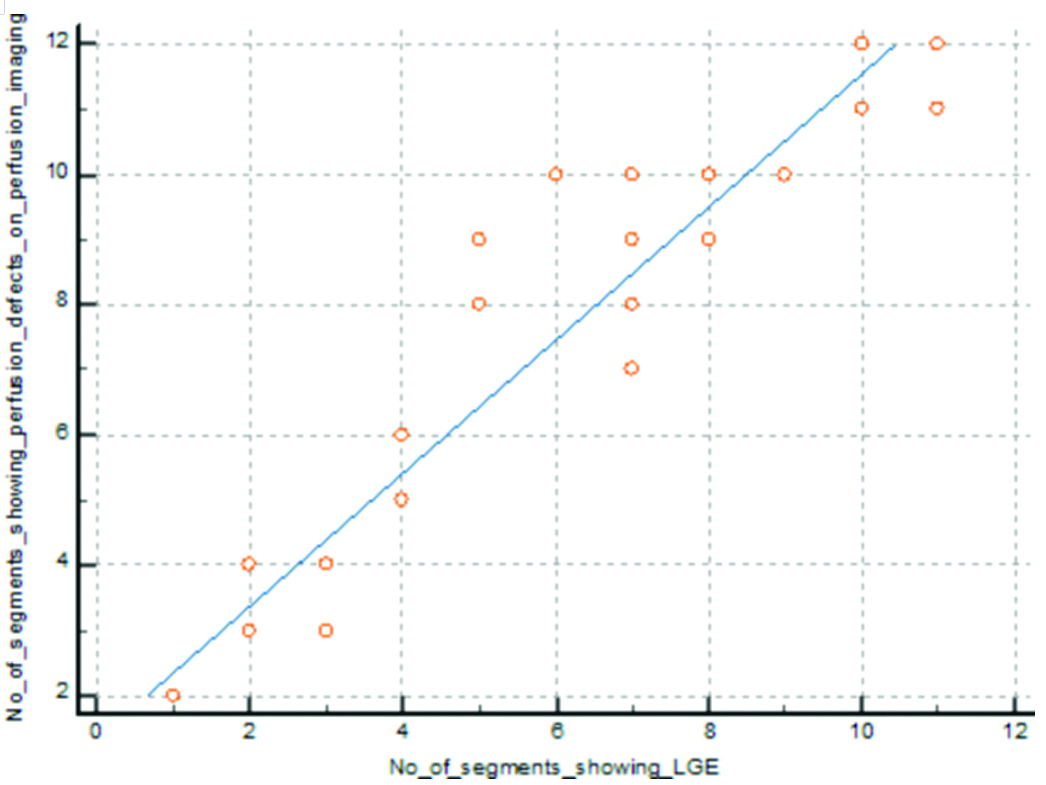
Out of 850 segments, 380 segments showed perfusion defects, and 307 segments showed LGE. There was a positive correlation between perfusion defect and LGE identified by cardiac MRI (p-value=0.0001) (Pearson’s correlation=0.951) [Table/Fig-13].
Correlation between perfusion defect and delayed enhancement in cardiac MRI.
Out of 50 patients, 17 patients showed an LGE score less than or equal to 50%, and 33 patients showed an LGE score more than 50%. All 17 patients who showed LGE score ≤50% underwent coronary revascularisation. Among the 33 patients who showed an LGE score >50%, 18 (55%) patients underwent coronary revascularisation based on clinical findings (those who had better chances of recovery), as well as, ECG and Echo findings, and the rest of the 15 (45%) patients were managed conservatively.
On follow-up after six months, with clinical evaluation and LVEF assessment by Echo, 15 (88.24%) out of 17 patients (who showed an LGE score ≤50%) showed improvement, as opposed to only 2 (11.11%) out of 18 patients with LGE score more than 50% who underwent coronary revascularisation. None of the patients of >50% LGE score group undergoing conservative treatment showed improvement [Table/Fig-14,15].
Distribution of segments showing LGE.
| Segments | LGE ≤50%n/n (%) | LGE >50%n/n (%) | Totaln/ n (%) |
|---|
| No. of segments showing LGE score 1 | 61 | 0 | 61 |
| No. of segments showing LGE score 2 | 63 | 0 | 63 |
| No. of segments showing LGE score 3 | 0 | 103 | 103 |
| No. of segments showing LGE score 4 | 0 | 80 | 80 |
| Total no. of segments showing LGE | 124 (40.3%) | 183 (59.6%) | 307 (100%) |
Distribution of outcome based on LGE.
| Distribution of LGE | Treatment | Total subjects | Improved | Not improved |
|---|
| n | % | n | % |
|---|
| LGE ≤50% | Coronary revascularisation | 17 | 15 | 88.24 | 2 | 11.76 |
| LGE >50% | Conservative | 15 | 0 | 0 | 15 | 100 |
| Coronary revascularisation | 18 | 2 | 11.11 | 16 | 88.89 |
A few representative images are shown in [Table/Fig-16,17,18 and 19].
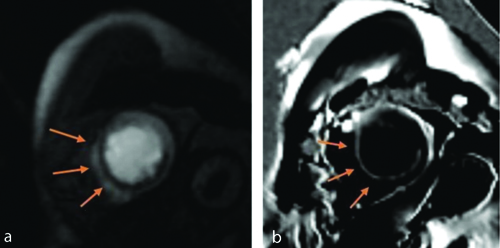
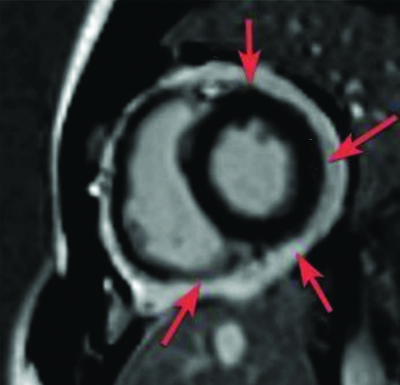
(a) Short axis images at left ventricular apex level. First pass gadolinium perfusion scan shows perfusion defects (arrows along apical anterior (13), apical septal (14), apical inferior (15) segments of left ventricular myocardium; b) On viability scan, delayed gadolinium contrast corresponding short axis image at left ventricular apex level shows <25% subendocardial LGE (arrows) along the same segments (13, 14 and 15) involving Right Coronary Artery (RCA) and Left Anterior Descending Artery (LAD) territories.
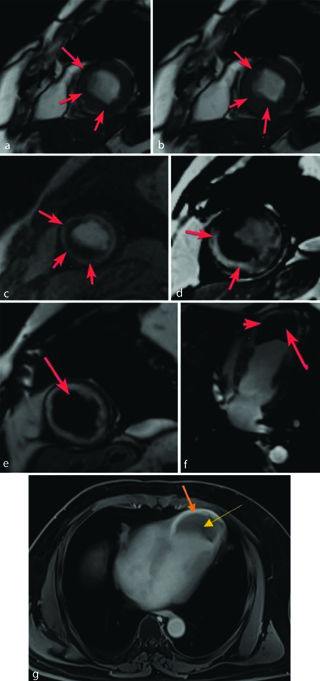
(a,b) End-diastolic (a) and end-systolic (b) frames of the short axis cine images at left ventricular apex level shows areas of moderate hypokinesia (arrows) along apical anterior (13), apical septal (14), apical inferior (15) segments of left ventricular myocardium; (c) First pass Perfusion short axis image perfusion defects (arrows) along the same myocardial segments; (d) Delayed gadolinium contrast short axis image shows 51-75% transmural LGE (arrows) involving LAD and RCA territories (LGE score 3); (e) White blood short axis image; and (f) White Blood vertical long axis image shows an apical aneurysm (arrow head) with a large thrombus (arrow) along with apical transmural enhancement; (g) Late gadolinium contrast horizontal long axis image; (g) shows apical aneurysm with large typically hypoenhancing thrombus (yellow arrow) along with apical transmural enhancement (orange arrow).
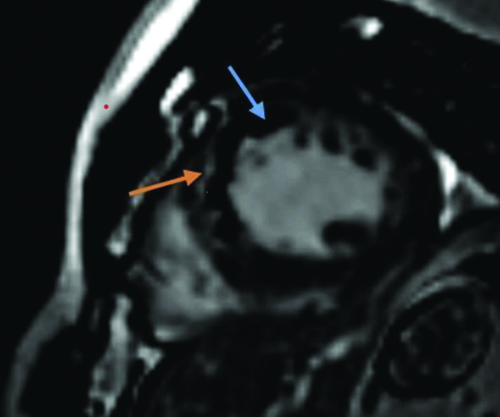
Late gadolinium scan short axis images at left ventricular mid cavity level shows a large area of microvascular obstruction as evidenced by sub-endocardial low signal (blue arrow) with overlying myocardial enhancement (orange arrow) along mid anterior septal (8) and mid inferior septal (9) segments of left ventricular myocardium.
Post gadolinium scan short axis images at left ventricular apex level shows diffuse enhancement of the visceral and parietal layers of pericardium (arrows), consistent with post MI pericarditis.
Case 1: LGE score ≤50% (LGE Score 1)
Case 2: LGE score >50% (LGE Score 3)
Case 3: LGE score >50% (LGE Score 4)
Case 4: LGE score >50% (LGE Score 4)
Discussion
The mean age of patients in the present study was 52±14 years, which correlated with the study by Shah V et al., where the mean age was 54.04 years [18]. Out of total, 27 (54%) patients were males and 23 (46%) patients were females. This finding correlated with the study by Sun W et al., where the majority of patients were male (67%) [19].
Dyslipidemia was found to be the most common co-morbidity (82% of study population), followed by smoking (78%), hypertension (76%), diabetes (74%), sedentary lifestyle (54%), obesity (52%), and stress (50%). In a study by Lee SA et al., diabetes was the most common co-morbidity (55%), followed by hypertension (47%), smoking (44%) and hyperlipidemia (32%) [20].
Out of 50 patients, ECG could detect an infarct in 40 (80%) patients. ECG could not detect an infarct in the remaining 10 (20%) patients. However, cardiac MRI could detect an infarct in all patients. Hence, cardiac MRI was found to be superior in detecting an infarct than ECG (p-value<0.05). It correlated with the study by Schelbert EB et al., where it was found that unrecognised myocardial infarction Unrecognised MI (UMI) detected by CMR (17%; 95% CI, 14% to 19%) was more prevalent than the UMI detected by ECG (5%; 95% CI, 4-6%; p-value<0.001) [21].
Out of 850 segments, 564 segments showed wall motion abnormality by Echo, and 606 segments showed wall motion abnormality detected by cardiac MRI. It correlated with the study by Kryukov NA et al., which showed that delayed contrast-enhanced cardiac MRI with cine sequences allowed to determine a higher number of impaired contractility cases compared with echocardiography (p-value=0.000006) [22].
Out of 850 segments, 380 segments showed perfusion defects, and 307 segments showed LGE. The number of segments showing perfusion defects exceeded the number of segments showing LGE (p-value=0.001). This correlated with the study by Sun W et al., where the segments with abnormal perfusion were more than in DE-MRI (p-value<0.05), which indicated that the area of abnormally perfused segments extended significantly beyond the region of infarction [15].
The mean ejection fraction of patients with an LGE score ≤50% (45±2%) was higher than the mean ejection fraction of patients with LGE score >50% (36±5%), which was found to be statistically significant (p-value=0.001). This correlated with the study by Lee SA et al., which showed that patients with LGE were more likely to have lower LVEF (p-value<0.05) [20].
It was seen that patients with an LGE score ≤50% benefited more with coronary revascularisation than patients with LGE score more than 50% (p-value<0.05). This is corroborated with the study by Kim RJ et al., where it was concluded that the chance of improvement in regional contractility following revascularisation gradually declined as the transmural extent of hyperenhancement before revascularisation rose (p-value=0.001) [11].
It also correlated with the study by Allman KC et al., which showed strong association between myocardial viability on noninvasive testing and improved survival after revascularisation in patients with chronic Coronary Artery Disease (CAD) and LV dysfunction (p-value<0.001) [23]. It also correlated with a study by Choi KM et al., it was found that improvement in segmental contractile function after coronary revascularisation was inversely related to the transmural extent of infarction on MRI (p-value=0.001) [12].
It also correlated with a study by Lim FY et al., it was found that the amount of myocardial viability, as measured by cardiac magnetic resonance LGE, appeared to distinguish individuals who benefit differently after CABG [24]. More non viable segments were significantly associated with higher All-cause Mortality (ACM) in the Coronary Artery Bypass Grafting (CABG) group (adjusted HR 1.19, 95% CI 1.01-1.41, p-value=0.036).
The LGE score is beneficial to stratify patients based on their treatment plans. Not all patients with chronic ischaemic heart disease benefit from coronary revascularisation. Myocardial viability is an important factor to determine the survival benefit of the patient after coronary revascularisation [18].
Infarcted or fibrotic tissue exhibits increased signal on T1-weighted sequences after the administration of the extracellular contrast agent as a result of reduced clearance and increased volume of distribution of the contrast agent in these regions as compared to normal myocardium, which appears dark. The ideal myocardial nulling time (T1 inversion time) is chosen to improve quantification of the infarcted bright zone [25].
Other methods used to test myocardial viability are: Single-photon Emission Computed Tomography (SPECT), Dobutamine Stress Echocardiography (DSE), Fluorodeoxyglucose F-18 (FDG) Positron Emission Tomography (PET) and invasive coronary angiography. But cardiac MRI has the following advantages over these methods: high-resolution imaging, high sensitivity and specificity, no radiation exposure, the ability to evaluate the transmural extent of scar tissue, and it is considered the gold standard for assessing LV volumes and EF [17].
Limitation(s)
There were few limitations of the study, one being its hospital-based subject selection, which had biases and issues, making it challenging to translate the findings to the general public. Additionally, the study’s potential sample size is less than desirable for the time frame.
Conclusion(s)
Cardiac MRI proves to be a vital modality for the non invasive determination of cardiac viability. Using the principles of LGE, with segments showing a score of more than 50% can be comprehensively tiered as non viable myocardium, thus providing a suitable roadmap for timely and appropriate intervention for patients suffering from MI. Cardiac MRI using the LGE score for viability testing is irrevocably beneficial for the management of patients with ischaemic heart disease.
DM: Diabetes mellitus; HTN: Hypertension