Introduction
Fissure-in-ano are a notably prevalent anorectal condition, primarily associated with ulcers and raised anal sphincter spasm. The management options largely focus on promoting wound healing and reducing anal spasm. Medical treatment, which includes a fibre-rich diet and sitz baths, results in the healing of nearly half of the fissure-in-ano. The ulcer of the anal canal is the presenting complaint in this condition. Topical Ayurvedic medicine can be utilised to heal such ulcers.
Need of the study
A review of the literature suggests that T. procumbens exhibits potent wound healing, antioxidant, antimicrobial, anti-inflammatory, procoagulant and immunomodulatory properties. Given that an acute fissure-in-ano presents as an ulcer in the anal canal, this study proposes the use of T. procumbens for its management.
Aim
To evaluate the efficacy of Jayanti (T. procumbens) cream versus topical Lignocaine and Nifedipine cream in the treatment.
Materials and Methods
A single-blind, double-arm comparative randomised control trial will be conducted at the Department of Shalyatantra, Mahatma Gandhi Ayurveda College Hospital and Research Centre (MGACHRC), Salod Hirapur (H), Maharashtra, India, from April 2023 to November 2024, with 70 subjects enrolled. In the control group, topical Lignocaine and Nifedipine cream will be applied, while in the interventional group, topical Jayanti (T. procumbens) cream will be applied to the acute fissure-in-ano. The evaluation parameters, including wound healing, anal spasm, per rectal bleeding, pain and itching, will be assessed.
Introduction
In surgical practice, fissure-in-ano is a benign, painful proctological ailment that affects overall well-being [1]. The probable understanding is that it results from a collective consequence of internal anal sphincter spasms, hard stools and various risk factors such as a high-fat diet, alcohol consumption, a non vegetarian diet, smoking and others; however, the exact cause of fissure-in-ano remains undetermined [2]. Typically, these fissure occur above the dentate line, with a higher prevalence in the midline posteriorly in males and anteriorly in females. In males, 95% of anal fissure are posterior and 5% are anterior, whereas in females, 80% are posterior and 20% are anterior [3]. The conservative approach is the initial line of management for fissure, aiming to normalise bowel movements through local medications that alleviate pain and irritation, along with the use of laxatives, stool softeners and a high-fibre diet. The optimal strategy for addressing the hypertonicity of the anal sphincter is a matter of debate, with potential options including medical or surgical intervention [4]. However, the majority of anal fissure respond to conservative management. One treatment approach may focus on achieving analgesia and promoting wound healing, which may help reduce the hypertonicity of the anal sphincter [5]. Lateral internal sphincterotomy is recognised as the most effective and well-established surgical method for managing fissure-in-ano, despite a recurrence rate that varies from 90% to 100% [6]. A notable drawback associated with lateral internal sphincterotomy is the potential onset of anal incontinence, primarily related to flatus [7]. Given the intricate nature of this condition, it is essential to identify effective, safe and promising treatment modalities within alternative sciences to facilitate early recovery and prevent recurrence. Although most studies suggest that the standard treatment for acute fissure-in-ano is the local application of Nifedipine and Lignocaine cream [8], the current scenario in India lacks comparative studies. Therefore, the present study aims to evaluate the efficacy of Jayanti (T. procumbens) cream versus topical Lignocaine and Nifedipine cream in the treatment of acute fissure-in-ano.
Primary objectives:
To study the efficacy of the healing effect of topical Jayanti (T. procumbens) cream in Parikartika (acute fissure-in-ano).
To study the efficacy of the healing effect of topical Lignocaine and Nifedipine cream in Parikartika (acute fissure-in-ano).
Secondary objectives: To compare the efficacy of the healing effect of topical Jayanti (T. procumbens) cream and Lignocaine and Nifedipine cream in the management of Parikartika (acute fissure-in-ano).
Null hypothesis: Topical Jayanti (T. procumbens) cream is not more efficacious than topical Lignocaine and Nifedipine cream in the management of Parikartika (acute fissure-in-ano).
Alternate hypothesis: Topical Jayanti (T. procumbens) cream is more efficacious than topical Lignocaine and Nifedipine cream in the management of Parikartika (acute fissure-in-ano).
Review of Literature
The effectiveness of conservative treatment for anal fissure is linked to the duration of symptoms. Therefore, acute anal fissure typically respond well to conservative treatments, which are considered the first line of therapy. Topical treatments available for anal fissure include calcium channel blockers like nifedipine and diltiazem, as well as nitrates like Nitroglycerin (NTG) ointment, Glyceryl Trinitrate (GTN), or its analogs like Isosorbide Dinitrate (ISDN). Additionally, topical anaesthetics like lignocaine are also used. However, the use of topical nitrate treatments is often limited due to adverse effects such as headaches, dizziness and postural hypotension [9].
In reviewing the literature, it is evident that one of the most common adverse effects of topical treatments for anal fissure is headache, particularly among subjects using diltiazem. Moreover, perianal dermatitis and hypotension have been reported due to diltiazem use. Nifedipine is better tolerated by subjects, exhibiting a lower incidence of adverse reactions compared to diltiazem [10]. Anal fissure are often associated with compromised blood supply to the anoderm due to elevated anal pressure. Nifedipine, a calcium channel blocker, addresses this issue by dilating blood vessels, thereby enhancing blood flow to the injured tissues. It also helps to lower the pressure in the internal anal sphincter, reducing pain and promoting healing. Lignocaine is primarily used for its anaesthetic properties [11].
T. procumbens is traditionally used by tribal communities for treating wounds. Research evaluating the antimicrobial properties of T. procumbens extracts revealed that both aqueous and petroleum ether extracts were effective in inhibiting the growth of bacterial strains such as Escherichia coli (E. coli) and Salmonella enterica serovar Typhimurium (S. typhimurium). The inhibitory effects of these extracts were comparable to those of the standard antibiotic ciprofloxacin at a concentration of 0.1 mg/mL. This study highlights the potential of T. procumbens as a source of bioactive compounds capable of combating bacterial infections [12].
In a study assessing the wound-healing properties of T. procumbens extract, the drug was dissolved in acetone and tested using a scratch assay. The results demonstrated the effectiveness of the extract at different concentrations, with the 40-μg concentration showing superior efficacy. Complete wound healing was observed by day 3 in this in-vitro study [13].
Another study indicated that out of 22 selected phytoconstituents, 18 demonstrated strong binding affinities. Notably, compounds such as beta-amyrone, betulinic acid, lupeol, stigmasterol, taraxasterol acetate and voacangine exhibited high binding affinities with most of the selected targets. These compounds are considered crucial due to their excellent synthetic accessibility. Beta-sitosterol is known for its potent antioxidant and anti-inflammatory properties, showing minimal toxicity and negligible ulcerogenic effects. Centaurein is noted for its anti-inflammatory effects via IFN-γ expression stimulation. Luteolin exhibits a range of beneficial properties, including antioxidant, anti-inflammatory, antiallergic and antitumor activities. Bergenin offers antimalarial, antihepatotoxic, anti-HIV, hepatoprotective, anti-inflammatory and immunomodulatory benefits and is a potential inhibitor against the main protease of SARS-CoV-2. Puerarin is recognised for its antioxidant and anti-inflammatory properties. Procumbentin stands out for its antinociceptive properties, making it the most promising compound identified in this study [14].
A study aimed at identifying and evaluating a serine protease from the aqueous extract of T. procumbens revealed its procoagulant and fibrinogenolytic properties, along with moderate fibrinolytic action. These findings suggest that the extract can form a haemostatic plug to stop bleeding from fresh cuts and subsequently initiate the dissolution of the fibrin clot, thereby promoting wound healing [15]. These findings emphasise the potential of T. procumbens in promoting wound healing and highlight its non toxic nature, presenting a promising option for managing advanced acute fissure-in-ano.
Materials and Methods
A single-blind, double-arm comparative randomised clinical trial will be conducted from April 2023 to November 2024 at the Outpatient Department (OPD) of Shalyatantra, Mahatma Gandhi Ayurveda Hospital, Wardha. The proposed study has received clearance under Institutional Ethical Committee (IEC) number MGACHRC/IEC/July-2022/568 and Clinical Trials Registry-India (CTRI) number CTRI/2023/03/050428. Those willing to enroll in the study will be considered upon providing informed written consent. Evaluation of assessment parameters, such as wound healing, spasm, per-rectal bleeding, pain and itching, will be conducted on days 0, 1, 7 and 15. Follow-up assessments are scheduled for the 30th day.
Inclusion criteria:
Subjects presenting with acute fissure-in-ano, initially diagnosed through rectal examination and with an onset timeframe of less than six weeks.
Individuals diagnosed with acute fissure-in-ano based on the 2019 ICD-10 CM Diagnosis Code K60.0 [16].
Age between 20 to 50 years, regardless of gender, occupation and socio-economic status.
Subjects exhibiting grade 0 and 1 anal spasm.
Controlled cases of Diabetes Mellitus (DM) (with Random Blood Sugar levels between 80 mg/dL and 140 mg/dL).
Known cases of immunocompromised conditions such as tuberculosis, Human Immunodeficiency Virus (HIV), hepatitis B and sexually transmitted diseases, who are receiving standard treatment.
Individuals willing to provide informed consent and prepared to adhere to clear directions.
Exclusion criteria:
Individuals falling outside the age range of 20 to 50 years.
Subjects with chronic fissure lasting more than six weeks and accompanied by a sentinel tag will be excluded.
Those with grade 2 anal spasms, as well as individuals experiencing conditions such as haemorrhoids, fistula-in-ano and anorectal carcinoma, will also be excluded.
Furthermore, subjects with uncontrolled diabetes mellitus (with Random Blood Sugar levels >140 mg/dL) and known cases of anaemia, bleeding disorders, Crohn’s disease and ulcerative colitis will be omitted from participation.
Sample size calculation: Cochran’s formula is used for sample size estimation, with a confidence level of (Z=1.67) and a margin of error (e=0.117), using a prevalence (p=0.1781) [17]. The sample size for this study is considered to be 35 in each group.
Study Procedure
Participants will be enlisted from the Shalyatantra OPD at Mahatma Gandhi Ayurveda Hospital, Wardha. Individuals who wish to participate in the study will be enrolled upon obtaining their written informed consent. Individuals diagnosed with acute fissure-in-ano will be included in the study using a computerised randomisation method. The recruitment of subjects will be conducted sequentially, adding one participant at a time until the predetermined target sample size is achieved. Participants who express a desire to discontinue during the trial will be allowed to withdraw and will be replaced accordingly. Additionally, if a patient develops an acute illness during the trial that could impact the study, the affected participant will be withdrawn and replaced.
Interventions: For the standard control treatment, group A, which includes the topical application of Lignocaine and Nifedipine cream twice a day following sitz baths, will be supplemented with Panchsakar Churna at a dosage of 5 grams (gm) at bedtime (HS) for 15 days.
For the interventional treatment, group B, which includes the topical application of Jayanti (T. procumbens) cream twice a day following sitz baths, will also be supplemented with Panchsakar Churna at a dosage of 5 gm at HS for 15 days.
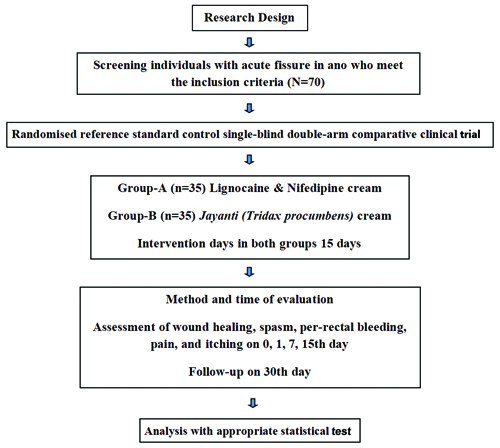
Follow-up will be conducted on the 30th day for both groups. [Table/Fig-1] depicts the study design, illustrating the grouping, intervention, assessment plan and follow-up.
Drug preparation: Fresh plants of T. procumbens will be collected from the campus of the study Institute and will be authenticated by the Department of Dravyaguna (Pharmacology and Materia Medica - Herbal).
Preparation of T. procumbens decoction extract: The T. procumbens decoction extract is prepared by combining one part of fresh T. procumbens plants with 16 parts of water, resulting in a proportion of 1/8th part for the final product through heat. This corresponds to using 1 kg of T. procumbens and 16 litres of water, yielding a total quantity of 2 litres for the T. procumbens decoction extract [18].
Preparation of T. procumbens paste (Kalka): A paste of fresh T. procumbens plants with a little water is created using a mortar and pestle, resulting in the formation of a paste. A paste weighing 125 mg will be prepared [19].
Preparation of T. procumbensGhrita (Ghee): The T. procumbensGhrita is formulated by combining one part paste, four parts Ghrita and 16 parts decoction extract, resulting in a proportion of 1/4th part for the final product through heat. This corresponds to using 125 mg of paste, 500 mg of Ghrita and two litres of decoction extract, yielding a total quantity of 412 mg for the T. procumbensGhrita [20].
Preparation of T. procumbens cream: The cream base is developed by blending ingredients; Batch-A’s composition includes 10% stearic acid, 10% cetostearyl alcohol, 5% pure white bees wax, 5% petroleum jelly, 30% water and 50% JayantiGhrita. This blend underwent continuous trituration and heating. In Batch A, the formulated cream will be stored for trial use [21]. Following the standardisation process, Batch A proved to be the preferred choice, displaying superior stability and consistency. The formulations of other batches are also stored and meticulously documented for future research purposes [Table/Fig-2].
Formulation of T. procumbens cream.

The medication is formulated at Dattatray Ayurveda Rasashala within the Mahatma Gandhi Ayurvedic College Hospital and Research Centre in Wardha, which holds a GMP certificate and operates as an Ayurvedic pharmacy. A commercially available topical formulation (Anobliss® Cream), containing a combination of Nifedipine 0.3% w/w and Lidocaine 1.5% w/w, will be purchased from the local market.
For controlled trials, subjects will be enlisted through a straightforward randomisation process utilising a computer-generated table. Envelopes used for this process will bear external serial numbers for identification. Essential details, including the patient ID and post-procedure results, will be documented.
Outcomes
Primary outcome [22]: Wound healing will be assessed using a grading assessment tool: 0 - completely healed fissure wound with a healthy scar; 1 - partially healed wound with granulation tissue; 2 - cleaned wound without slough/discharge; 3 - wound with discharge. Anal spasms will be evaluated using a grading assessment tool: 0 - normal (one finger can be passed); 1 - finger can be passed with severe pain; 2 - no finger can be passed.
Secondary outcome: Per rectal bleeding will be assessed as follows: 0 - no bleeding; 1 - mild (<5 drops); 2 - moderate (5-10 drops); 3 - severe (>10 drops) [22,23]. Pain will be assessed using the Visual Analogue Scale (VAS) (1-10): 0 - no pain; 1 - mild (1-3); 2 - moderate (4-6); 3 - severe (7-9); 4 - unbearable pain (10) [24]. Itching will be assessed using the Numerical Rating Scale: 0 - no itch or disturbance; 10 - worst imaginable itch or disturbance [25]. Improvements in the primary outcomes, including wound healing, anal spasms, per rectal bleeding, pain and itching, are anticipated as enhancements in the clinical features of acute fissure-in-ano.
Statistical Analysis
Statistical analysis will be conducted using Statistical Package for Social Sciences (SPSS) statistical software version 29.0. Student’s paired t-test will be used to compare the pre-treatment and post-treatment values within the intra-group, assessing changes in wound healing, anal spasm, per rectal bleeding, pain and itching over time. The Unpaired t-test will be used to evaluate the differences in outcomes for wound healing, anal spasm, per rectal bleeding, pain and itching between groups. A p-value of <0.05 will be considered significant.
[1]. Gardner IH, Siddharthan RV, Tsikitis VL, Benign anorectal disease: Hemorrhoids, fissures, and fistulasAnn Gastroenterol 2020 33(1):09-18.Epub 2019 Nov 2910.20524/aog.2019.043831892792PMC6928486 [Google Scholar] [CrossRef] [PubMed]
[2]. Kad AM, Akhtar M, Sonarkar R, Saxena D, Kumar K, Keswani S, A comparison of segmental internal sphincterotomy versus lateral internal sphincterotomy in management of chronic fissure in anoInt Sur J 2017 4(9):3044-48.Available from: https://doi.org/10.18203/2349-2902.isj2017388410.18203/2349-2902.isj20173884 [Google Scholar] [CrossRef]
[3]. Sriram Bhat M, SRB’s Manual of Surgery, Rectum and Anal Canal 2013 New DelhiJaypee Brothers Medical Publishers (P) Ltd:1046 [Google Scholar]
[4]. Lund JN, Scholefield JH, Aetiology and treatment of anal fissureBr J Surg 1996 83(10):1335-44.Available from: https://doi.org/10.1002/bjs.180083100610.1002/bjs.18008310068944447 [Google Scholar] [CrossRef] [PubMed]
[5]. Zaghiyan KN, Fleshner P, Anal fissureClin Colon Rectal Surg 2011 24(1):22-30.10.1055/s-0031-127282022379402PMC3140330 [Google Scholar] [CrossRef] [PubMed]
[6]. Jonas M, Scholefield JH, Anal fissureGastroenterol Clin North Am 2001 30(1):167-81.10.1016/s0889-8553(05)70172-211394029 [Google Scholar] [CrossRef] [PubMed]
[7]. Rather SA, Dar TI, Malik AA, Rather AA, Khan A, Parray FQ, Subcutaneous Internal Lateral Sphincterotomy (SILS) versus nitroglycerine ointment in anal fissure: A prospective studyInt J Surg 2010 8(3):248-51.Epub 2010 Feb 1310.1016/j.ijsu.2010.01.01320156605 [Google Scholar] [CrossRef] [PubMed]
[8]. Singh B, Khichy S, Kumar A, Singh S, Neki NS, Comparative study to observe effects of topical nifedipine with lignocaine and topical sucralfate with lignocaine in acute anal fissureJ Adv Med Dent Scie Res 2016 4(6):81-85.10.21276/jamdsr.2016.4.6.18 [Google Scholar] [CrossRef]
[9]. Emile SH, Elgendy H, Elfeki H, Magdy A, Abdelmawla AA, Abdelnaby M, Does the duration of symptoms of anal fissure impact its response to conservative treatment? A prospective cohort studyInt J Surg 2017 44:64-70.Available from: https://doi.org/10.1016/j.ijsu.2017.06.04410.1016/j.ijsu.2017.06.04428629768 [Google Scholar] [CrossRef] [PubMed]
[10]. Kujur ADS, Paul Ekka NM, Chandra S, Lal S, Malua S, Comparative study to assess the effectiveness of topical nifedipine and diltiazem in the treatment of chronic anal fissureJ Family Med Prim Care 2020 9(11):5652-57.10.4103/jfmpc.jfmpc_986_2033532409PMC7842453 [Google Scholar] [CrossRef] [PubMed]
[11]. Patel JR, Dudhamal TS, A comparative clinical study of Yashtimadhu Ghrita and lignocaine-nifedipine ointment in the management of Parikartika (acute fissure-in-ano)Ayu 2017 38(1-2):46-51.10.4103/ayu.AYU_93_1729861592PMC5954261 [Google Scholar] [CrossRef] [PubMed]
[12]. Behera S, Mahalik G, A survey on the ethnomedicinal plants and antimicrobial activity of Tridax procumbens LResearch Journal of Pharmacy and Technology 2022 15(1):219-23.10.52711/0974-360X.2022.00036 [Google Scholar] [CrossRef]
[13]. Tripathi N, Makode D, Jawade V, Verma M, Kanwar JR, Chowdhary R, Exploring the wound healing potential of Tridax procumbens extract: A comprehensive analysis through in vitro cytotoxicity and scratch assay studiesSSR Inst Int J Life Sci 2024 10(2):5146-51.10.21276/SSR-IIJLS.2024.10.2.20 [Google Scholar] [CrossRef]
[14]. Sharbidre A, Dhage P, Duggal H, Meshram R, In silico investigation of Tridax procumbens phyto-constituents against SARS-CoV-2 infectionBiointerface Res Appl Chem 2021 11(4):12120-148.Available from: https://doi.org/10.33263/BRIAC114.121201214810.33263/BRIAC114.1212012148 [Google Scholar] [CrossRef]
[15]. Gubbiveeranna V, Kusuma CG, Bhavana S, Sumachirayu CK, Ravikumar H, Nagaraju S, Potent procoagulant and platelet aggregation inducing serine protease from Tridax procumbens extractPhcog Res 2019 11(4):363-70.10.4103/pr.pr_4_19 [Google Scholar] [CrossRef]
[16]. World Health OrganizationChapter XI Diseases of the digestive system (K00-K93). International Statistical Classification of Diseases and Related Health Problems 2019 Geneva, Switzerland[cited 2024 Jul 06]. Available from: https://icd.who.int/browse10/2019/en#/K55-K64 [Google Scholar]
[17]. Chaudhary R, Dausage CS, Prevalence of anal fissure in subjects with anorectal disorders: A single-centre experienceJ Clin Diagn Res 2019 13(2):PC05-PC07.Available from: https://www.doi.org/10.7860/JCDR/2019/38478/1256310.7860/JCDR/2019/38478.12563 [Google Scholar] [CrossRef]
[18]. Bhatt NS, Deshpande M, A critical review and significance of ayurvedic preparation Kwatha - Herbal decoctionInternational Journal of Ayurvedic Medicine 2020 11(2):155-64.Available from: https://doi.org/10.47552/ijam.v11i2.149510.47552/ijam.v11i2.1495 [Google Scholar] [CrossRef]
[19]. Gohil H, Dhruve K, Prajapati PK, Role of media in the preparation of Apamarga KsharatailaAyu 2010 31(3):391-94.10.4103/0974-8520.7715822131746PMC3221078 [Google Scholar] [CrossRef] [PubMed]
[20]. Bhatt N, Deshpande M, A critical review and significance of lipid-based ayurvedic dosage forms Ghrita and Taila: Part I-review and statusInt J Curr Pharm Res 2023 15(2):07-16.Available from: https://dx.doi.org/10.22159/ijcpr.2023v15i2.209110.22159/ijcpr.2023v15i2.2091 [Google Scholar] [CrossRef]
[21]. Ashok Babu VL, Madriwala B, Jeevitha L, Mathew MS, Abhilash S, Singh A, Development, characterization and in vitro antifungal evaluation of topical formulation of Tridax procumbens L. Leaf extractJ Pharmacogn Phytochem 2022 11(2):289-95.Available from: https://doi.org/10.22271/phyto.2022.v11.i2d.1439410.22271/phyto.2022.v11.i2d.14394 [Google Scholar] [CrossRef]
[22]. Badwe Y, Pendam K, Study the effect of chandanbalalakshadi taila pichu in parikartika with special reference to fissure-in-ano: A pilot studyAyushdhara 2020 7(1):2545-52.Available from: https://ayushdhara.in/index.php/ayushdhara/article/view/517 [Google Scholar]
[23]. Shinde J, Mugave B, Badwe Y, Pilot study on efficacy of Sarjarasa malahara local application in Parikartika with special reference to Fissure-in-anoInternational Journal of Ayurvedic Medicine 2022 13(1):254-57.Available from: https://doi.org/10.47552/ijam.v13i1.240810.47552/ijam.v13i1.2408 [Google Scholar] [CrossRef]
[24]. Heller GZ, Manuguerra M, Chow R, How to analyze the visual analogue scale: myths, truths and clinical relevanceScand J Pain 2016 13:67-75.10.1016/j.sjpain.2016.06.01228850536 [Google Scholar] [CrossRef] [PubMed]
[25]. Ikoma A, Ebata T, Chantalat L, Takemura K, Mizzi F, Poncet M, Measurement of nocturnal scratching in subjects with pruritus using a smart watch: Initial clinical studies with the itch tracker AppActa Derm Venereol 2019 99(3):268-73.10.2340/00015555-310530523352 [Google Scholar] [CrossRef] [PubMed]