Tuberculosis (TB) continues to be a major global health issue, necessitating effective treatment regimens primarily involving antitubercular drugs. First-line antitubercular drugs are commonly used in combination with other medications to treat TB. These drugs are essential for the treatment of TB, a contagious bacterial infection caused by Mycobacterium tuberculosis. However, antitubercular drugs can lead to various skin reactions, which may range from mild to severe. Stevens-Johnson Syndrome (SJS) is a rare and severe reaction characterised by extensive skin peeling and mucosal involvement. It can occur with any antitubercular drug but is more commonly associated with isoniazid and rifampicin. Rifampicin, an essential medication in the treatment of TB, is known for its efficacy but also for its potential to cause severe adverse reactions. The spectrum of skin reactions can vary widely, from benign rashes to Severe Cutaneous Adverse Reactions (SCARs), like SJS. Early recognition and appropriate management are crucial to mitigate complications. Understanding these adverse effects is essential not only for immediate patient care but also for fostering long-term adherence to TB treatment, as fear of side effects can lead patients to abandon their regimens prematurely. Hereby, the authors present a rare case of a 55-year-old male who developed life-threatening skin reactions, including SJS, following the initiation of rifampicin therapy. Rapid recognition and intervention were critical, leading to the immediate discontinuation of rifampicin and the initiation of supportive care and corticosteroids. The patient was closely monitored, and his condition improved significantly over the following weeks, with gradual resolution of skin lesions and restoration of mucosal integrity.
Case Report
A 55-year-old male patient, a construction worker by profession and a chronic smoker, was diagnosed with pulmonary tuberculosis and started on Isoniazid (H), Rifampicin (R), Ethambutol (E), and Pyrazinamide (Z). He presented to the Department of Respiratory Medicine with skin lesions covering his entire body, associated with itching and dryness of the lips, within seven days of starting Antitubercular Treatment (ATT). Hyperpigmented lesions were present throughout the body. The lesions began on the neck and progressed to the chest, abdomen, back, and bilateral upper and lower limbs, eventually involving the face and accompanied by dryness of the lips within a period of three days.
Similar lesions had appeared five months ago following ATT with a first-line fixed-dose combination, after which treatment was stopped, and the skin lesions subsided within seven days. The patient had left the treatment and did not complete the course. Recently, he was again diagnosed with microbiologically confirmed TB and again after starting ATT, developed similar skin lesions with itching all over the body within seven days.
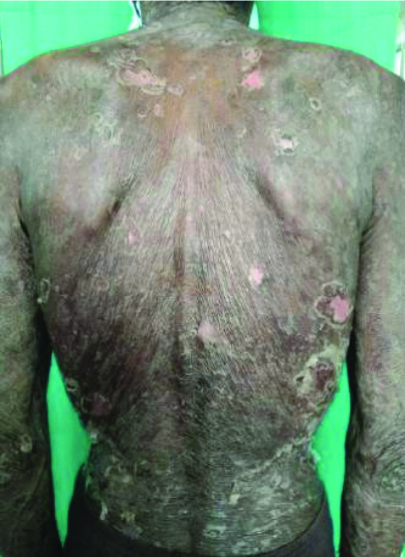
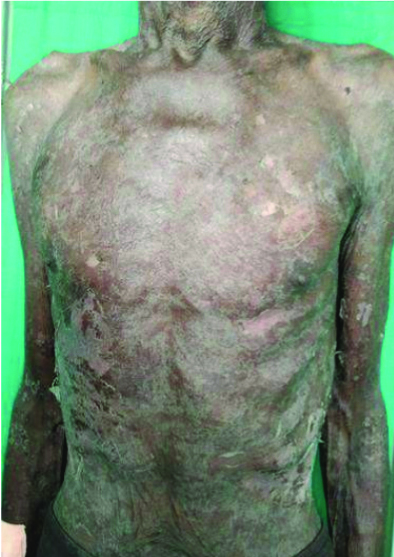
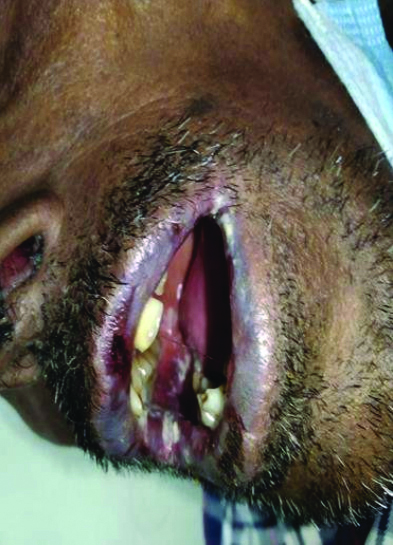
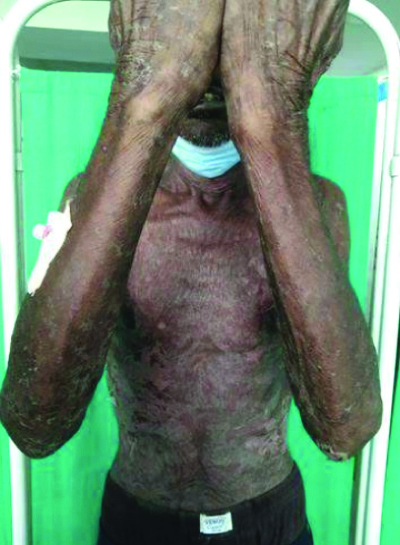
On examination, multiple erythematous to hyperpigmented patches were observed over the nape of the neck, bilateral arms, bilateral forearms, chest, abdomen, back, bilateral thighs, bilateral lower limbs, and buttocks. The palms and soles were also involved. Haemorrhagic crusts were noted on the upper and lower lips [Table/Fig-1,2,3 and 4]. The Nikolsky sign was positive over the upper limbs and back. The patient was provisionally diagnosed with Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (TEN) overlap syndrome. Other differential diagnosis included drug reaction, erythema multiforme major, and Staphylococcal Scalded Skin Syndrome (SSSS).
Multiple erythematous and hyperpigmented patches with scaling over the neck and back.
Multiple erythematous and hyperpigmented patches with scaling over the chest and abdomen.
Haemorrhagic crusting of bilateral upper and lower lips.
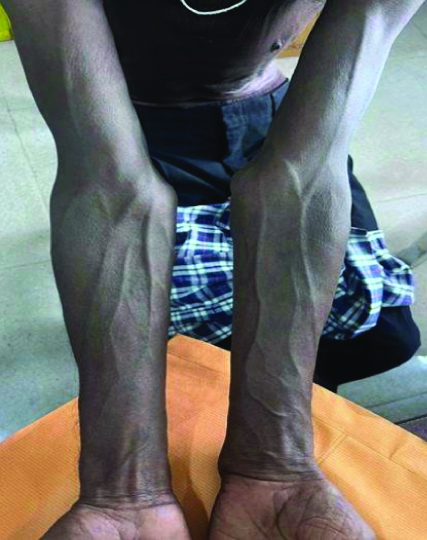
Hyperpigmentation over upper limbs.
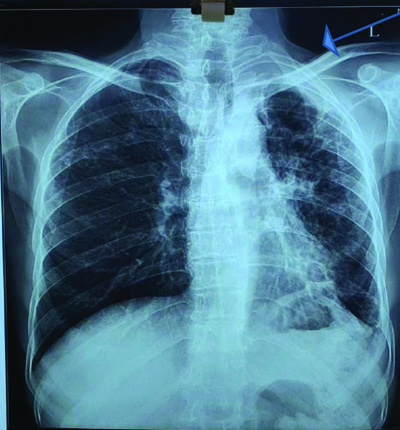
Recently, he was again diagnosed with microbiologically confirmed TB and, after starting ATT, developed similar skin lesions with itching all over his body within seven days. He presented to the hospital with a fever (temperature: 100.6°F), blood pressure of 130/100 mm Hg, oxygen saturation of 98% on room air, and a heart rate of 86 beats/min. Laboratory examinations showed normal haemoglobin, leucocyte count, and platelet levels, as well as normal liver enzymes (serum alanine transaminase and serum aspartate transaminase) [Table/Fig-5]. A chest X-ray revealed a cavitary lesion in the left upper zone [Table/Fig-6].
| Laboratory findings | Values |
|---|
| Hb | 10.4 g/dL |
| TLC | 10,500 cells/microliter |
| N/L | 68/15% |
| E/M | 3/3% |
| Platelets | 4,50,000/microliter |
| PT/INR | 1.07 |
| RBS | 105 mg/dL |
| Urea | 30 mg/dL |
| Sr. Creat | 1.1 mg/dL |
| T. Bil/D/ID | 0.3/0.1/0.2 mg/dL |
| SGOT | 24 U/L |
| SGPT | 27 U/L |
Hb: Haemoglobin; TLC: Total leucocyte count; N: Neutrophils; L: Lymphocytes; E: Eosinophil; M: Monocyte; PT: Prothrombin time; INR: International normalised ratio; RBS: Random blood sugar; Sr. Creat: Serum creatinine; T. Bil: Total bilirubin; D: Direct bilirubin; ID: Indirect bilirubin; SGOT: Serum glutamic-oxaloacetic transaminase; SGPT: Serum glutamate pyruvate transaminase; g/dL: Grams per decilitre; mg/dL: Miligrams/decilitre; U/L: Units per litre
Chest X-ray showing left upper zone’s cavitary lesions.
The standard antitubercular regimen was promptly discontinued due to a suspected allergic reaction. The patient received treatment with antihistamines, steroids, intravenous fluids, electrolyte supplementation, fusidic acid cream lotion, and calamine lotion as per the dermatologist’s advice, leading to symptom improvement. Chlorhexidine mouth gargles were prescribed three times a day, along with the local application of a 0.1% steroid for oral lesions for 30 minutes after meals.
When the lesions had healed, an individual antitubercular drug was started to identify the culprit drug. First-line ATT was initiated sequentially, beginning with the drug least likely to cause a hypersensitivity reaction, starting at the lowest dose and gradually increasing to the appropriate dose, with ongoing monitoring. Initially, ethambutol was introduced at an initial dose of 100 mg. The dose was gradually increased to 600 mg without any hypersensitivity reactions. Next, isoniazid was reintroduced at a dose of 50 mg and slowly increased to 200 mg, with no symptoms reported. Pyrazinamide was then reintroduced at 250 mg and gradually escalated to 1000 mg without any adverse reactions. Symptoms and vitals were regularly monitored and remained stable.
Rifampicin was introduced at a dose of 150 mg. However, within five days of starting rifampicin, the patient developed a hypersensitivity reaction, presenting as a rash on the upper and lower limbs, accompanied by itching. Clinically, based on the drug history and examination of the skin lesions, he was diagnosed with SJS. As there was no history of any viral infections and target lesions on the skin, erythema multiforme was deemed unlikely. SSSS involves the rapid development of a tender erythematous desquamative rash on the face and flexures, followed by the formation of large, fragile, potentially purulent blisters. The absence of bacteremia in routine blood investigations ruled out SSSS.
The condition was managed as an anaphylactic reaction with antihistamines and a short course of steroids, following which the symptoms resolved. The hypersensitivity reaction was attributed to rifampicin, and the patient was discharged on isoniazid, pyrazinamide, ethambutol, and levofloxacin, with follow-up scheduled.
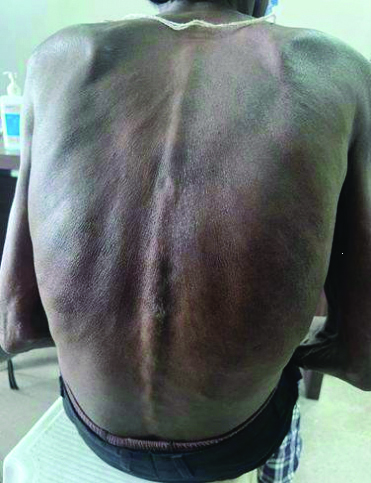
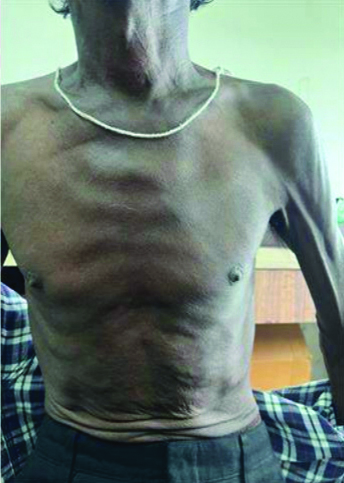
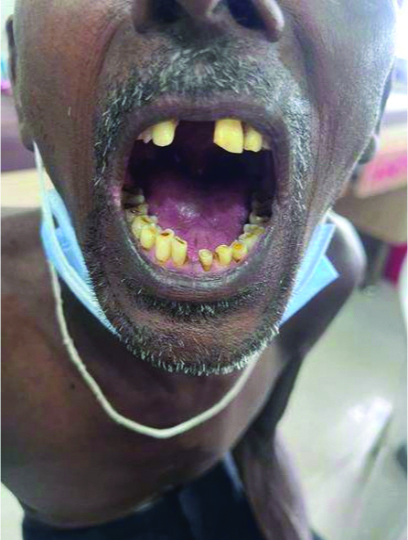
On follow-up, the patient was symptomatically better. The hyperpigmented lesions and mucosal ulcerations had completely resolved. [Table/Fig-7,8,9 and 10].
Healed lesions over neck and back after treatment.
Healed lesions over chest and abdomen.
Healed oral ulcers and resolution of the upper limb’s haemorrhagic crusts.
Resolution of bilateral hyperpigmentation.
Discussion
The SJS is a serious allergic reaction that affects the skin and mucous membranes, causing skin damage and cracking. It is painful and can be fatal. Usually, SJS begins eight days (with a range of 4 to 30 days) after starting ATT-Isoniazid, Rifampicin, Ethambutol, and Pyrazinamide. Antitubercular medicines are a prominent source of SJS and TEN in underdeveloped nations such as India [1].
A 0.6% to 7.5% of patients experience Cutaneous Adverse Drug Reactions (CADRs) during ATT [2]. CADRs can range in severity, and although there are no universally accepted grading systems, mild reactions typically involve simple rashes or itching. Moderate reactions may include more widespread skin issues, such as blistering. Severe reactions, including Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), SJS, TEN, drug hypersensitivity syndrome, cutaneous vasculitis, and Fixed Drug Eruptions (FDEs), can be life-threatening and often require hospitalisation [3]. The drug re-challenge test is widely regarded as the gold standard for identifying the offending drug, helping to minimise interruptions in ATT [4]. If, a patient develops a skin reaction while on antitubercular therapy, it is important to assess the severity and consider whether the reaction is likely related to the medication [5]. Most adverse drug reactions (75-80%) are due to predictable, non immunologic effects of drugs. The remaining 20-25% result from unpredictable effects, which may or may not be immune-mediated. Immune-mediated reactions represent 5-10% of all drug reactions [6]. Rifampicin is the most likely anti-TB medication to be implicated as a culprit agent. Management may involve discontinuing the offending drug and potentially replacing it with an alternative treatment if, necessary [7]. Following the introduction of Fixed-Dose Combination (FDC) ATT in India in 2016, which transitioned from intermittent therapy to a daily regimen based on the patient’s body weight, a slight increase in the incidence of drug reactions was noted. This increase may be attributed to several factors, including better TB detection rates, improved treatment adherence, earlier identification of CADRs, or the potentially higher daily drug dosages compared to the previous thrice-weekly regimen [8].
In present case, Rifampicin was identified as the drug responsible for the ATT-induced cutaneous drug reaction. Similarly, in the study by Rizzi A et al., among antitubercular drugs, rifampicin was the most common drug involved, followed by isoniazid, in the development of DRESS [9]. Allouchery M et al., reported the largest series of antitubercular drug-associated DRESS, describing 67 cases [10]. They recorded rifampicin and isoniazid as the most involved drugs, with a median time of symptoms onset of 24 days of treatment. According to a study conducted in Turkey by Katran ZY et al., 8% of people worldwide experience drug hypersensitivity reactions to antituberculosis medications. Type IV hypersensitivity reactions, which usually take the form of maculopapular eruptions, occurred in about one-third of the patients [11]. The most common drugs associated with these reactions were rifampicin, followed by pyrazinamide, isoniazid, and ethambutol.
The present case highlights the potential for severe cutaneous reactions to the antitubercular drug Rifampicin, despite its effectiveness as part of TB therapy. Early recognition and discontinuation of the offending drug are crucial for managing such adverse reactions. In present case, prompt action and supportive care led to a favourable outcome.
Conclusion(s)
Antitubercular drug-induced skin reactions are a critical aspect of TB treatment that require awareness and proactive management. Understanding the mechanisms, prevention strategies, and the latest research insights helps mitigate these adverse effects and ensures effective and safe treatment for TB. Vigilance and timely intervention are essential in managing these adverse effects to prevent serious complications and ensure successful treatment of TB. Regular updates in clinical practice guidelines and ongoing research are essential for improving patient outcomes and minimising the risk of severe skin reactions.
Hb: Haemoglobin; TLC: Total leucocyte count; N: Neutrophils; L: Lymphocytes; E: Eosinophil; M: Monocyte; PT: Prothrombin time; INR: International normalised ratio; RBS: Random blood sugar; Sr. Creat: Serum creatinine; T. Bil: Total bilirubin; D: Direct bilirubin; ID: Indirect bilirubin; SGOT: Serum glutamic-oxaloacetic transaminase; SGPT: Serum glutamate pyruvate transaminase; g/dL: Grams per decilitre; mg/dL: Miligrams/decilitre; U/L: Units per litre
[1]. Priyadarshini I, Thole A, Hazarika K, Ghadlinge MS, An observed repeat case of DRESS syndrome with modified anti-tuberculosis therapy-a case reportInt J Adv Med 2024 11:130-34.10.18203/2349-3933.ijam20240365 [Google Scholar] [CrossRef]
[2]. Beytout Q, Godefroy N, Monsel G, Jaureguiberry S, Caumes E, Clinical management of anti-tuberculosis related cutaneous adverse drug reactions based on reintroductionJ Travel Med 2023 30(8):taad134Available from: https://doi.org/10.1093/jtm/taad13410.1093/jtm/taad13437831914 [Google Scholar] [CrossRef] [PubMed]
[3]. Bhattarai HB, Yadav J, Sapkota S, Adhikari A, Bhattarai M, Singh I, Cutaneous drug reaction secondary to antitubercular regimen: A case report from NepalSAGE Open Med Case Rep 2023 11:2050313X23121039010.1177/2050313X23121039037954539PMC10637153 [Google Scholar] [CrossRef] [PubMed]
[4]. Lee JY, Seol YJ, Shin DW, Kim DY, Chun HW, Kim BY, A case of the Drug Reaction with Eosinophilia and Systemic Symptom (DRESS) following isoniazid treatmentTuberc Respir Dis (Seoul) 2015 78(1):27-30.10.4046/trd.2015.78.1.2725653694PMC4311032 [Google Scholar] [CrossRef] [PubMed]
[5]. Khan MMZ, Shah SH, Lee HK, Adverse effects of antitubercular medications and their managementAmerican Journal of Respiratory Critical Care Medicine 2021 203(12):1583-93. [Google Scholar]
[6]. Sharma RK, Verma GK, Tegta GR, Sood S, Rattan R, Gupta M, Spectrum of cutaneous adverse drug reactions to anti-tubercular drugs and safe therapy after re-challenge- A retrospective studyIndian Dermatol Online J 2020 11(2):177-81.10.4103/idoj.IDOJ_133_1932477975PMC7247648 [Google Scholar] [CrossRef] [PubMed]
[7]. Patel LM, Robinson AJK, Dermatological reactions to systemic antitubercular therapyBritish Journal of Dermatology 2022 186(4):625-33. [Google Scholar]
[8]. Gupta G, Das AK, Kirtana J, Baitha U, Sinha S, Drug-induced hypersensitivity reaction and re-introduction of Anti-Tubercular Drugs (ATT): A case report and review of literatureJ Drug Delivery Ther 2023 13(6):01-05.10.22270/jddt.v13i6.6080 [Google Scholar] [CrossRef]
[9]. Rizzi A, Nucera E, Urbani S, Longhino D, Lohmeyer FM, Gambassi G, Antituberculosis drug-induced non-blistering systemic severe reactions: A 10-year (2012-2022) literature reviewAsian Pac J Allergy Immunol 2023 41(4):273-91.Available from: https://doi.org/10.12932/ap-010423-158210.12932/AP-010423-1582 [Google Scholar] [CrossRef]
[10]. Allouchery M, Logerot S, Cottin J, Pralong P, Villier C, Ben Saïd B, French Pharmacovigilance Centers Network and the French Investigators for skin adverse reactions to drugsAntituberculosis drug-associated DRESS: A case seriesJ Allergy Clin Immunol Pract 2018 6(4):1373-80.Epub 2017 Dec 2010.1016/j.jaip.2017.11.02129274824 [Google Scholar] [CrossRef] [PubMed]
[11]. Katran ZY, Bulut I, Babalik A, Keren M, Treatment and management of hypersensitivity reactions developed against anti-tuberculosis drugInt J Mycobacteriol 2022 11(3):309-17.10.4103/ijmy.ijmy_78_2236260451 [Google Scholar] [CrossRef] [PubMed]