The HCV infection is a liver disease caused by the HCV. It is one of the major risk factors leading to acute and chronic conditions such as altered liver function, the development of chronic hepatitis, cirrhosis, hepatocellular carcinoma and liver failure. HCV infection poses a significant health risk to cancer patients, particularly in regions with variable prevalence rates [1]. Understanding the prevalence and associated factors of HCV infection in this population is crucial for effective management and prevention strategies. Previous studies have highlighted the importance of routine screening for blood-borne viruses in oncology settings to mitigate the risk of transmission and ensure timely interventions [2]. However, the prevalence of HCV infection among cancer patients can vary depending on geographical location, patient demographics and healthcare practices [3]. In regions like Gujarat, India, where resources may be limited and rural populations are prevalent, the burden of HCV infection among cancer patients warrants further investigation.
Insufficient HCV screening in developing countries like India means that infections from various sources, such as unscreened blood donors, intravenous drug use and unsafe injection practices, will continue to contribute to the disease burden [4-6]. A study conducted by Patel PH et al., reported a prevalence rate of HCV infection of 0.28% among cancer patients attending a tertiary care hospital in Valsad, Gujarat, India. This highlights the need for localised studies to understand the regional epidemiology of HCV infection in cancer patients [7]. Additionally, advancements in cancer treatment modalities, such as chemotherapy and immunosuppressive therapies, may further complicate the management of HCV infection in this vulnerable population.
Furthermore, to date, there is no vaccine available for hepatitis C infection; therefore, the prevention of HCV infection relies solely on reducing the risk of exposure [4]. Consequently, comprehensive knowledge of the prevalence, risk factors and clinical characteristics of HCV infection among cancer patients is essential for optimising patient care and implementing preventive measures. While there are many studies available on the prevalence of HCV in the general patient population, present study was focused specifically on cancer patients.
Thus, the aim of this study was to determine the prevalence of HCV infection in patients attending a cancer care hospital in Ahmedabad, Gujarat, India.
Materials and Methods
This cross-sectional study was conducted in the Department of Microbiology at The Gujarat Cancer and Research Institute (GCRI), a tertiary care cancer hospital in Gujarat, India. The study was carried out from August 2018 to December 2019, spanning a period of 17 months. All patients who attended GCRI during the study period were included in the study, as it was time-bound. This study received approval from the Scientific and Ethics Committee of the Institute, and approval from the Institutional Review Board was granted (GCRI no. 5842-17/07/18).
Inclusion criteria: Patients admitted for cancer treatment in the fields of medicine, paediatrics, surgery, gynaecology, and neuro-oncology were included in the study.
Exclusion criteria: Previous HCV-reactive patients were excluded from the study.
All demographic details were recorded and a detailed proforma was filled out for each patient. Potential risk factors associated with HCV infection were assessed [6-8]. Blood samples were collected from all patients at the time of admission to the hospital and were transferred to the microbiology laboratory using universal standard transport guidelines.
Upon arrival at the laboratory, all samples were subjected to HCV testing by ELISA after centrifugation. The samples were tested using the ELISA on a fully automated ELISA system (Euphoria 4.1, Tulip Diagnostics). The Qualisa HCV (Tulip Diagnostics) kit was used for the detection of anti-HCV [8]. All positive samples were tested twice to confirm the results according to the guidelines set by the kit [Table/Fig-1].
Reporting policy of HCV-ELISA.
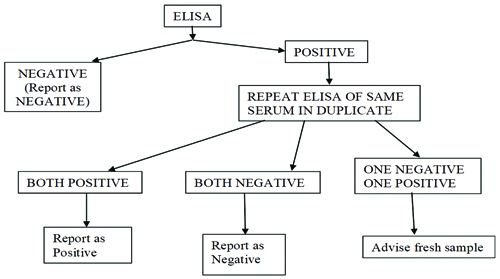
For quality control, the Internal Quality Control (IQC) was run daily along with kit controls. The Levey-Jennings Chart (LJ Chart) of IQCs were created monthly.
Statistical Analysis
The prevalence was expressed as the number of cases with a positive test result divided by the total number of cases tested. Statistical analysis was performed using SPSS (SPSS Inc., Chicago, IL, version 13.0) and Epi-Info (version 3) statistical software.
Results
During the study period from August 2018 to December 2019, a total of 45,886 blood samples were received for HCV infection screening. Out of these samples tested, 190 (0.41%) were found to be HCV positive [Table/Fig-2]. [Table/Fig-3,4] show the demographic details of the HCV positive patients. The demographic analysis indicated that the mean age was around 50 years for both sexes. HCV positivity was observed equally in both genders. The prevalence of HCV was higher among adult patients living in rural areas. The highest prevalence of HCV was observed in the age group of 51-60 years, with 35 cases (18.42%) in females and 29 cases (15.26%) in males.
Seroprevalence of HCV infection in cancer patients.
| Prevalence of HCV infection | Number of samples |
|---|
| HCV negative | 45696 |
| HCV positive | 190 (0.41%) |
| Total samples | 45886 |
Demographic data of HCV Patients 190 (0.41%).
| Demographic data | n (%) |
|---|
| Gender |
| Female | 95 (50) |
| Male | 95 (50) |
| Age-wise |
| Paediatric (≤14 years) | 10 (5.3) |
| Adult (>14 years) | 180 (94.7) |
| Residence |
| Urban | 34 (17.9) |
| Rural | 156 (82.1) |
Age and sex wise distribution of patients having HCV infection.
| Age group (years) | Female n (%) | Male n (%) |
|---|
| 1-10 | 6 (3.16) | 3 (1.58) |
| 11-20 | 0 | 3 (1.58) |
| 21-30 | 2 (1.05) | 4 (2.11) |
| 31-40 | 13 (6.84) | 13 (6.84) |
| 41-50 | 18 (9.48) | 18 (9.47) |
| 51-60 | 35 (18.42) | 29 (15.26) |
| 61-70 | 16 (8.42) | 19 (10) |
| 71-80 | 5 (2.63) | 6 (3.16) |
| Total | 95 (50) | 95 (50) |
| Mean age | 50.56 | 50.96 |
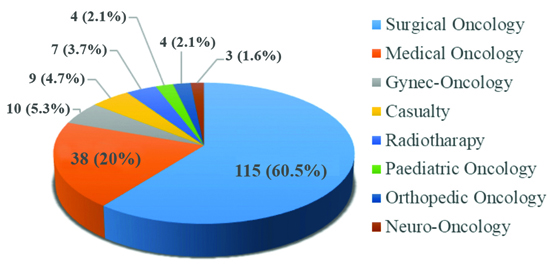
Out of 190 HCV-positive patients, 176 (92.6%) had non haematological malignancies (solid tumours), while the remaining 14 (7.4%) had haematological malignancies [Table/Fig-5]. During the study of the 190 HCV-positive patients, 115 (60%) were diagnosed in the surgical oncology department, 38 (20%) in the medical oncology department, and the rest were from gynaecologic oncology, neuro-oncology, etc., [Table/Fig-6].
HCV positive cancer patients in haematological Vs non haematological cancer.
| S. No. | Type of malignancy | HCV n (%) |
|---|
| 1 | Haematological cancer | 14 (7.4) |
| 2 | Non Haematological cancer176 (92.6%) | Digestive system | 112 (58.9) |
| Endocrine system | 1 (0.5) |
| Exocrine system | 10 (5.3) |
| Lymphatic node | 3 (1.6) |
| Nervous system | 14 (7.4) |
| Renal system | 4 (2.1) |
| Reproductive system | 13 (6.8) |
| Respiratory system | 9 (4.7) |
| Skeletal system | 8 (4.2) |
| Unspecified carcinoma | 2 (1.1) |
| Total | 190 (100) |
Unit wise distribution of HCV positive.
The majority of cancers among HCV-positive patients were related to the digestive system, accounting for nearly 112 cases (58.9%). Other notable cancer types include those affecting the reproductive system (13 cases, 6.8%), and nervous system (14 cases, 7.4%). Additionally, cancers of the skeletal system (8 cases, 4.2%), exocrine system (10 cases, 5.3%), and respiratory system (9 cases, 4.7%) were observed. Cancers affecting the immune and lymphatic systems (3 cases, 1.6%), renal system (4 cases, 2.1%), and endocrine system (1 case, 0.5%) were less common among HCV-positive patients. While cancers of the skeletal, exocrine, and respiratory systems have been observed, they represent smaller percentages of the total cases. Cancers affecting the immune, renal, and endocrine systems are relatively rare, with each contributing less than 3% of the total cases [Table/Fig-5].
Different types of risk factors associated with HCV transmission are presented in [Table/Fig-7]. The most common risk factor identified was transmission through blood transfusion, accounting for 35 cases (18.4%).
Risk factors for HCV infection in cancer patients (N=190).
| Personal history wise distribution of HCV | Before admission in hospital n (%) | After admission in hospital n (%) | Grand total n (%) |
|---|
| Blood transfusion | 27 (16.0) | 8 (38.1) | 35 (18.4) |
| Unsafe sex | 6 (3.6) | 0 | 6 (3.2) |
| Minor OT | 8 (4.7) | 1 (4.8) | 9 (4.7) |
| Post radiotherapy | 22 (13.0) | 9 (42.9) | 31 (16.3) |
| Tattoo mark | 3 (1.8) | 1 (4.8) | 4 (2.1) |
| Major OT | 2 (1.2) | 1 (4.8) | 3 (1.6) |
| Family history | 1 (0.6) | 0 | 1 (0.5) |
| Negative history of above factors | 100 (59.2) | 1 (4.8) | 101 (53.2) |
| Total | 169 (100) | 21 (100) | 190 (100) |
Discussion
As per the GCRI institute policy, all patients admitted for diagnosis and treatment underwent preliminary diagnostic tests along with screening for blood borne viruses. During the 17-month study period, from August 2018 to December 2019, the seroprevalence of HCV infections among cancer patients at GCRI was 0.41%. In contrast, Vohra P et al., reported a similar prevalence rate of 0.68% in their study [9]. In India, various studies have reported different prevalence rates ranging from 0.09 to 2.02% [10]. Thus, the prevalence of HCV in present study was quite similar to those conducted by others. Patel PH et al., reported a 0.28% HCV infection rate among patients attending a tertiary care hospital in Valsad, Gujarat, which was lower than present study. This discrepancy may be due to the immunocompromised status of the patients [7].
The analysis of demographic data revealed an equal distribution of HCV infection between genders, with females and males each accounting for 95 (50%) of the total HCV-positive cases, which totaled 190 (0.41%). This was comparable to the findings of Patel PH et al., [7]. Niu ZL et al., also observed that there was no significant difference in the overall percentage between men and women over the past five years [11]. However, a significant predominance of adults (>14 years) was observed, comprising 180 (94.7%) of the HCV-positive population, compared to only 10 (5.3%) paediatric cases (≤14 years). The study conducted by Chowdhury A et al., indicates an increase in HCV prevalence with age, suggesting a steady cumulative rise in the incidence of the infection [12]. This implies a higher prevalence of HCV infection among adults, indicating a potential age-related susceptibility to the virus.
Regarding residential distribution, the majority of HCV-positive individuals resided in rural areas, comprising 156 (82.1%) of the sample, compared to 34 (17.9%) in urban areas. Similar findings were reported by Sood A et al., who observed a higher prevalence of anti-HCV among rural residents compared to urban residents, reinforcing the predominance of HCV-positive individuals in rural areas [13]. This finding highlights a possible association between HCV infection and rural living conditions. Rural populations may face unique challenges in accessing healthcare services for HCV prevention, diagnosis and treatment compared to urban populations. Further research is warranted to explore the underlying factors contributing to the higher prevalence of HCV infection in rural areas.
Regarding cancer types among HCV-positive patients, the most prevalent cancer was of the digestive system, accounting for 112 cases (58.9%), followed by cancers of the reproductive system with 13 cases (6.8%). These findings are consistent with those of Omland LH et al., who identified 51 cases of Gastrointestinal Tract (GIT) cancer, five cases of reproductive system cancer, and four cases of hepatological cancers out of a total of 100 cases [14]. These results corroborate previous studies suggesting a potential link between HCV infection and certain types of cancer, particularly hepatocellular carcinoma.
Piazzolla AV et al., reported that among the 356 HCV Ab-positive individuals, only 182 (51.1%) were associated with surgical units. In contrast, among the 114 HCV RNA-positive cases, 59 (51.8%) were linked to surgical units, with 24 (54.5%) of the 44 HCV RNA-positive cases being unaware of their infection. These findings align with present study, which also observed a higher prevalence of HCV-positive cases in surgical oncology units (115 cases, or 60%) compared to medical oncology units (38 cases, or 20%) [15]. This underscores the importance of screening and managing HCV infection among cancer patients undergoing treatment.
Personal history-wise distribution indicated that blood transfusion (35 cases, 18.4%) and radiotherapy (31 cases, 16.3%) were the most common factors associated with HCV infection before and after admission to the hospital, respectively. Niu ZL et al., found that blood transfusion (18.14%) was the primary route of transmission for HCV in their study [11]. A history of blood transfusion was reported by 13.6% of participants in a study conducted by Petruzziello A et al., [16]. However, a significant proportion of HCV-positive individuals-103 cases (54.2%)-had no history of the aforementioned risk factors, suggesting the possibility of other modes of transmission or undiagnosed routes.
Key strategies to reduce HCV transmission include [17]:
Promoting injection safety and infection control in healthcare settings, recommending the use of Auto-disable (AD) syringes.
Ensuring the safety of blood and blood products through routine screening.
Reducing the demand for unnecessary transfusions.
Recognising that the epidemiology of HCV infection is not well characterised in many countries; special studies and enhanced surveillance activities are needed to refine prevention strategies and monitor the impact of prevention efforts.
Regular cleaning and disinfection of endoscopes, gastro scopes, and laparoscopes with ortho-phthalaldehyde must be performed according to established guidelines.
WHO recommendations [17]:
Promote infection control through the adoption of national guidelines and an accreditation process to monitor compliance.
Conduct necessary studies and surveillance activities to better understand the epidemiology of hepatitis C in selected countries.
Implement comprehensive training programs for Healthcare Practitioners (HCPs), as these are fundamental tools in preventing the transmission of blood-borne pathogens.
Limitation(s)
The sample size of the present study was small as it was time-bound. Further studies are needed to collect a larger number of samples to estimate the different epidemiologies of HCV. Additionally, the present study did not assess the extent of nosocomial transmission occurring in the current setting; therefore, future studies can be conducted with this in mind.
Conclusion(s)
Routine screening for viral agents such as Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), and HCV is recommended for all patients with malignancies before starting cancer treatments that lead to immunosuppression, such as chemotherapy and radiotherapy. Addressing the transmission of these infections requires educational campaigns and screening efforts tailored to vulnerable populations, including collaboration with healthcare providers to reach illiterate communities. Further studies on the seroprevalence of various diseases in the community can be valuable in formulating new policies to combat these infections.