Beta-thalassaemia is an autosomal recessive disease resulting from point mutations in the beta-globin gene, leading to either its absence or a decrease in production [1]. Out of the two types, beta-thalassaemia major (Cooley’s anaemia) is the most severe type. It is associated with severe anaemia, often with haemoglobin levels falling below 7 g/dL [2]. In low socio-economic regions, such as India, the primary management approach for beta-thalassaemia major often involves regular packed red blood cell transfusions, effective iron chelation therapy, and addressing complications related to iron overload [3]. Iron overload is a significant complication associated with repeated transfusion therapy, the excess of which starts accumulating in organs, mainly the liver, resulting in fibrosis [4]. As time progresses, it further impacts the heart and other tissues [5]. Additionally, increased gastrointestinal iron absorption exacerbates the transfusional iron burden, saturating liver proteins such as transferrin and ferritin [6]. Due to frequent transfusions, patients with beta-thalassaemia are at a greater risk for viral infections, especially Hepatitis C [7,8]. In beta-thalassaemia, both iron overload and Hepatitis C Virus (HCV)-related liver disease lead, through different mechanisms, to hepatocellular necrosis, fibrosis, and cirrhosis [9].
As liver fibrosis represents a substantial share of morbidity in multi-transfused thalassaemia patients, early assessment and prompt intervention can significantly halt the progression to full-blown cirrhosis. Liver biopsy is currently the gold standard for assessing structural changes in liver architecture, but it has its limitations, with invasiveness being the most significant [10]. Moreover, it is a costly and often painful procedure, and since it samples only a portion of the liver tissue, it may not accurately reflect the severity of fibrosis across the entire liver [11]. There is also the potential for sampling errors, along with inter- and intraobserver variability, and the risk of complications [12]. TE has emerged as another promising technique that measures the speed of an elastic wave passing through the liver [13].
The FIB-4 and APRI are some of the simple non invasive methods for assessing liver fibrosis that have been used with varying degrees of accuracy as surrogates to measure alterations in liver architecture. These indices can be easily calculated from routine haematological investigations [14]. They were originally validated in chronic viral hepatitis B and C, as well as in Non-alcoholic Fatty Liver Disease (NAFLD), with emerging evidence in other liver disorders [15].
The YKL-40 is another rapidly emerging biomarker of inflammation. YKL-40, also known as Chitinase-3-like protein 1 (CHI3L1), is a glycoprotein involved in inflammation and tissue remodeling. It is primarily produced by macrophages, neutrophils, and various cells in connective tissue, and it plays a role in cellular proliferation, differentiation, and angiogenesis. Elevated levels of YKL-40 are associated with diseases involving inflammation and fibrosis, including liver fibrosis, cardiovascular disease, and some cancers [9].
Studies have highlighted the utility of non invasive liver fibrosis biomarkers in various hepatic conditions, including NAFLD, chronic hepatitis (B and C), biliary atresia, and cirrhosis [16-21]. While some studies have assessed liver fibrosis in beta-thalassaemia major using tools like TE, there is a gap in research specifically examining the role of non invasive biomarkers such as FIB-4, APRI, and YKL-40 in this condition [22,23]. These biomarkers have been valuable in other liver diseases but remain underexplored in beta-thalassaemia major. Therefore, the present study was planned to fill this gap by evaluating the diagnostic value of non invasive liver parameters, such as FIB-4, APRI, and YKL-40, as potential early markers of liver fibrosis by correlating them with TE in children with beta-thalassaemia major receiving regular transfusions.
Materials and Methods
The present cross-sectional study was conducted in the Department of Paediatrics, along with the Department of Hepatology and Gastroenterology, at Pt. BD Sharma Postgraduate Institute of Medical Sciences, Rohtak, Haryana, India, from January 2020 to February 2022, after obtaining approval from the Institutional Ethical Committee (BREC/19/157). The study participants were recruited from the paediatric day care facility after obtaining written informed consent from the parents or Legally Acceptable Representatives (LAR) of the children.
Inclusion criteria: Children diagnosed with beta-thalassaemia major in the age group of 5 to 18 years.
Exclusion criteria: Children with chronic viral hepatitis, other known haemophilias or coagulation disorders, chronic liver diseases (e.g., Wilson’s disease, autoimmune hepatitis), and co-morbid conditions like Chronic Kidney Disease (CKD), coeliac disease, diabetes, and tuberculosis were excluded from the study.
Sample size calculation: A convenient sampling method was adopted, as the present study was initiated as a small-scale investigation. A total of 75 children were enrolled in the study.
Study Procedure
After taking a thorough history emphasising demographic characteristics, disease duration, frequency of transfusions, and the iron chelation regimen—including type, dose, duration, and compliance—each participant underwent a detailed clinical assessment, including anthropometric measurements and a complete systemic examination.
Analysis of biochemical parameters: A 5 mL venous blood sample was collected under aseptic precautions for biochemical analysis. Routine biochemical investigation like complete haemogram, viral markers, liver function tests, renal function tests and lipid profile were undertaken. Ferritin levels were measured using an indirect Enzyme-Linked Immunosorbent Assay (ELISA) kit (Orgentec, Germany) [24]. Ferritin levels were categorised as <1000 ng/mL, 1000-3000 ng/mL, 3000-5000 ng/mL, and >5000 ng/mL for structured analysis [25]. The remaining samples were stored at -80°C for subsequent analysis. Fibrosis scores (FIB-4 and APRI) were calculated. FIB-4 was calculated using the formula: FIB-4=Age {(yr)×AST (U/L)}/{(PLT (10 (9)/L))×(ALT (U/L)) (1/2)} [14]. The APRI was calculated using the following formula: APRI=(AST/Upper Limit of Normal AST)×100/Platelet count (109/L) [26]. Serum YKL-40 levels were measured by a commercially available sandwich Enzyme linked Immunosorbent assay YKL-40 ELISA kit (Elabscience Biotech Inc.) [27]. Liver Stiffness Measurements (LSN) were conducted using TE via FibroScan 502 for all participants by a single experienced gastroenterologist who was blinded to the clinical and laboratory details of the children. With the patient lying in dorsal decubitus, probe was placed on the skin overlying right lobe of liver. A median of 10 values were recorded for each subject, with the results being presented in kilopascals (kPa). Values obtained on TE were converted into a corresponding METAVIR score and the results were mapped to an F0-F4 scale using validated cut-off values: ≤7.0 kPa for F0-F1, 7.1-9.5 kPa for F2, 9.6-12.5 kPa for F3, >12.5 kPa for F4 [28]. Only scores with an interquartile range of ≤30% were deemed reliable for study inclusion.
Statistical Analysis
Statistical analysis was performed using SPSS software version 20.0. The data were analysed using appropriate statistical methods and p-values. The Shapiro-Wilk test was applied to assess the normality of the variables. For normally distributed (parametric) variables, the t-test was used, while the Wilcoxon-Mann-Whitney U test was employed for non normally distributed (non parametric) variables. Correlations between variables were explored using non parametric tests (Spearman’s correlation). Statistical differences were assessed with the Chi-square test, with a p-value <0.05 considered statistically significant and <0.001 considered highly significant.
Results
The mean age of the patients was 10.71±2.54 {mean±Standard Deviation (SD)} years, with a median age of 11 years and an age range of 6 to 16 years. Out of 75 children, 54 (72%) were male, and 21 (28%) were female. All participants (100%) were on chelation therapy. The average weight and height of the children were 30.91±10.52 kg and 133.91±11.89 cm, respectively. The mean frequency of blood transfusions was 16.51±1.43 days [Table/Fig-1].
Summary of baseline characteristics.
| Parameters | Mean±SD |
|---|
| Age (years) | 10.71±2.54 |
| Gender (n%) |
| Male | 54 (72.0%) |
| Female | 21 (28.0%) |
| Time of diagnosis (Months of age) | 5.95±1.05 |
| Chelators (yes) (n%) | 75 (100.0%) |
| Weight (kg) | 30.91±10.52 |
| Height (cm) | 133.91±11.89 |
| Frequency of transfusion (days) | 16.51±1.43 |
The analysis of diagnostic markers revealed that the mean APRI was 0.27±0.14, while the mean value for FIB-4 was 0.58±0.28. The YKL-40 levels had a mean of 889.40±1205.92 pg/mL. The LSN using TE showed a mean value of 5.13±1.82 kPa [Table/Fig-2].
| Markers | Mean±SD | Median (IQR) |
|---|
| APRI | 0.27±0.14 | 0.24 (0.19-0.31) |
| FIB-4 | 0.58±0.28 | 0.46 (0.39-0.67) |
| YKL-40 (pg/mL) | 889.40±1205.92 | 300 (160-735) |
| LSN (kPa) | 5.13±1.82 | 4.40 (3.90-5.60) |
Out of a total of 75 children, 46 individuals (61.3%) exhibited ferritin levels below 1000 ng/mL. Total 17 (22.7%) children had ferritin levels ranging from 1000 to 3000 ng/mL, 9 (12.0%) children had levels between 3000 and 5000 ng/mL, and 3 (4.0%) children presented with ferritin levels exceeding 5000 ng/mL [Table/Fig-3]. Total 62 (82.7%) children exhibited a fibrosis grade of F0-F1, signifying minimal to no fibrosis. Total 12 (16.0%) children were categorised as F2, reflecting moderate fibrosis, whereas 1 (1.3%) was classified with a fibrosis grade of F3, indicating severe fibrosis [Table/Fig-4].
Distribution of participants’ ferritin levels (N=75).
| Ferritin | Frequency (n) | Percentage (%) |
|---|
| <1000 ng/mL | 46 | 61.3 |
| 1000-3000 ng/mL | 17 | 22.7 |
| 3000-5000 ng/mL | 9 | 12.0 |
| >5000 ng/mL | 3 | 4.0 |
Distribution of the participants in terms of LSN fibrosis grade (N=75).
| LSN fibrosis grade | Frequency (n) | Percentage (%) |
|---|
| F0-F1 (Little to no fibrosis) | 62 | 82.7 |
| F2 | 12 | 16.0 |
| F3 | 1 | 1.3 |
The analysis of LSN in kPa across different ferritin levels showed a statistically significant (p<0.001) increase in liver stiffness with higher ferritin levels. The group with ferritin levels >5000 ng/mL had the highest mean LSN of 8.73±0.60 kPa, with a median of 8.8 kPa (IQR: 8.45-9.05 kPa) [Table/Fig-5]. Similarly, the range of YKL-40 levels also increased significantly (p<0.001) with higher ferritin levels, ranging from 65-2000 pg/mL in the group with ferritin levels below 1000 ng/mL to 3400-4000 pg/mL in the group with ferritin levels exceeding 5000 ng/mL [Table/Fig-6].
Comparison of the four subgroups of the variable ferritin in terms of LSN (kPa) (N=75).
| LSN (kPa) | Ferritin | p-value |
|---|
| <1000 ng/mL | 1000-3000 ng/mL | 3000-5000 ng/mL | >5000 ng/mL |
|---|
| Mean±SD | 4.26±0.72 | 5.68±2.02 | 7.37±2.02 | 8.73±0.60 | <0.001 |
| Median (IQR) | 4.3 (3.8-4.5) | 4.7 (4.3-7.1) | 8.2 (5.9-9.1) | 8.8 (8.45-9.05) |
| Range | 3.1-6.8 | 3.2-9.4 | 4.2-9.5 | 8.1-9.7 |
Comparison of the four subgroups of the variable ferritin in terms of YKL-40 (pg/mL) (N=75).
| YKL-40 (pg/mL) | Ferritin | p-value |
|---|
| <1000 ng/mL | 1000-3000 ng/mL | 3000-5000 ng/mL | >5000 ng/mL |
|---|
| Mean±SD | 345.54±342.81 | 1078.82±1273.32 | 2368.89±1512.72 | 3716.67±301.39 | <0.001 |
| Median (IQR) | 250 (150-437.5) | 400 (200-2100) | 3000 (670-3600) | 3750 (3575-3875) |
| Range | 65-2000 | 140-3800 | 100-3700 | 3400-4000 |
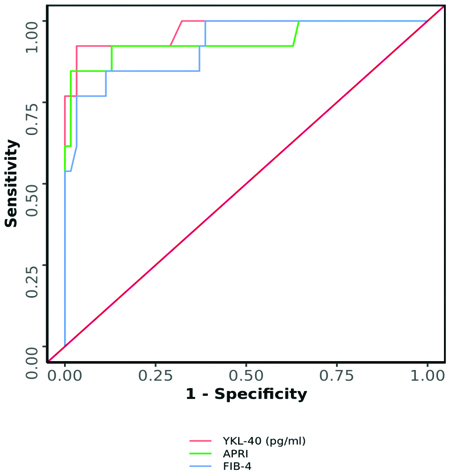
A statistically significant moderate positive correlation was observed between LSN (kPa) and YKL-40 (pg/mL) (Spearman’s r=0.6; p<0.001) [Table/Fig-7]. A moderate positive correlation was found between FIB-4 and YKL-40 levels (Spearman’s r=0.3; p=0.003) [Table/Fig-8]. In contrast, a strong positive correlation was noted between APRI and YKL-40 (Spearman’s r=0.8; p<0.001) [Table/Fig-9], APRI and LSN (kPa) (Spearman’s r=0.8; p<0.001) [Table/Fig-10], and FIB-4 and LSN (kPa) (Spearman’s r=0.7; p<0.001) [Table/Fig-11]. The AUROC for YKL-40, APRI, and FIB-4 was 0.971, 0.937, and 0.926, respectively. YKL-40 exhibited a maximum sensitivity of 92%, while APRI showed a maximum specificity of 98% [Table/Fig-12,13]. APRI demonstrated the best positive predictive value (96.78%), while YKL-40 (pg/mL) had the highest negative predictive value (99.01%). Both YKL-40 and APRI demonstrated the best diagnostic accuracy (91.21% and 90.67%, respectively), making them equally reliable in distinguishing between positive and negative cases [Table/Fig-14].
Correlation between LSN (kPa) and YKL-40 (pg/ml) (N=75).
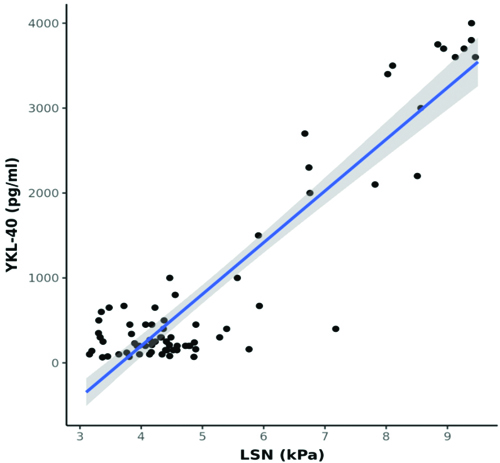
Correlation between FIB-4 and YKL-40 (pg/mL) (N=75).
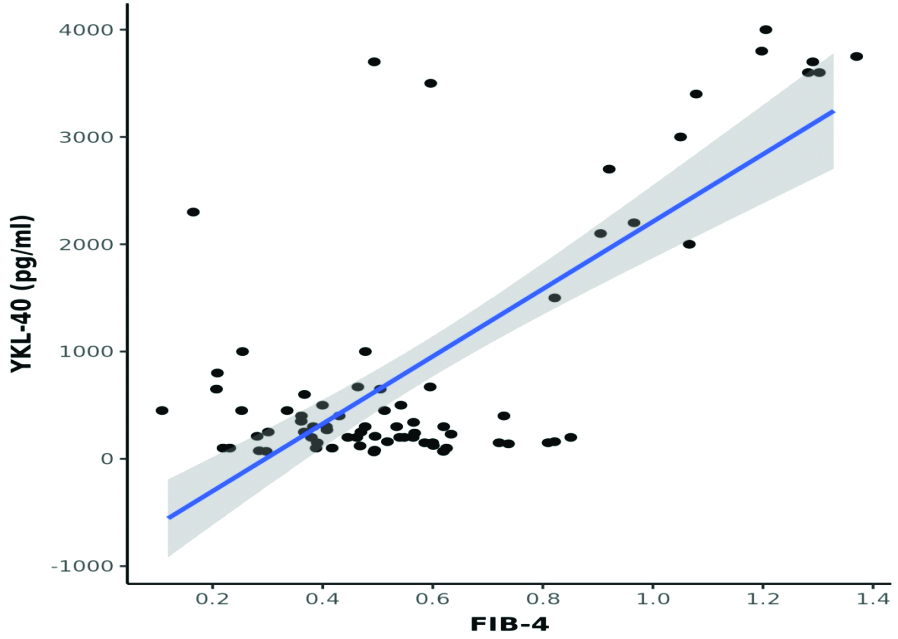
Correlation between APRI and YKL-40 (pg/mL) (N=75).
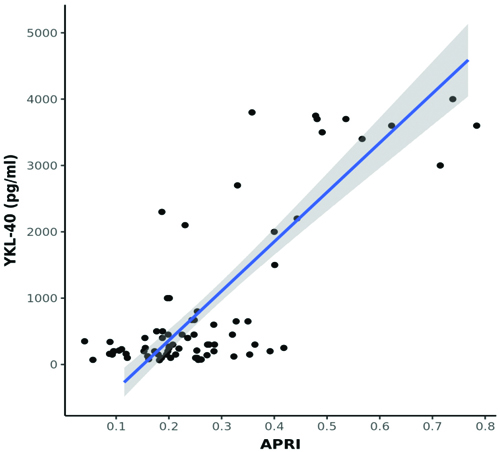
Correlation between APRI and LSN (kPa) (N=75).
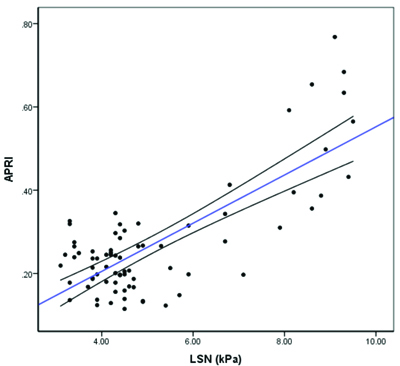
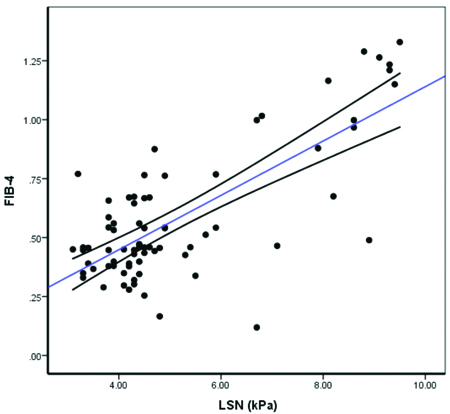
Correlation between FIB-4 and LSN (kPa) (N=75).
Comparison of the diagnostic performance of various predictors in predicting fibrosis on LSN: yes vs fibrosis on LSN: No.
| Predictor | AUROC | 95% CI | p-value | Sensitivity | Specificity |
|---|
| YKL-40 (pg/mL) | 0.971 | 0.924-1 | <0.001 | 92% | 97% |
| APRI | 0.937 | 0.84-1 | <0.001 | 85% | 98% |
| FIB-4 | 0.926 | 0.846-1 | <0.001 | 77% | 97% |
YKL-40 (pg/mL), APRI, and FIB-4 significantly predicted fibrosis on LSN: Yes.
Positive Predictive Values (PPVs), Negative Predictive Values (NPVs), and diagnostic accuracies of YKL-40 (pg/mL), APRI, and FIB-4.
| Predictor | Positive Predictive Value (PPV) | Negative Predictive Value (NPV) | Diagnostic accuracy |
|---|
| YKL-40 (pg/mL) | 92.31% | 99.01% | 91.21% |
| APRI | 96.78% | 98.75% | 90.67% |
| FIB-4 | 88.57% | 95.11% | 89.33% |
Discussion
The present study found that non invasive markers of liver fibrosis (APRI, FIB-4, and YKL-40) are reliable tools for detecting early liver fibrosis in children with beta-thalassaemia compared to traditional methods. Among the various non invasive markers, YKL-40 (pg/mL) was the most reliable for sensitivity, negative predictive value, and diagnostic accuracy, while APRI exhibited the highest specificity and positive predictive value. The mean age±SD of patients enrolled in present study was 10.71±2.54 years, which was lower compared to a previous study [29]. In the present study, the gender distribution showed a male predominance. This was similar to a study conducted by Pipaliya N et al., where out of 154 enrolled, 99 (64%) were males and 55 (36%) were females [30]. The mean frequency of transfusions was once every 16-20 days, which was quite comparable to studies conducted previously. There was no significant difference in LSN between the two genders. This finding contrasts with a previous study by Prati D et al., which reported an increased risk of liver fibrosis in males [31]. In present study, age had a significant impact on the development of fibrosis, with older patients being at a higher risk of liver fibrosis, likely due to a greater number of transfusions and insufficient chelation. This finding contrasts with a study by Al-Khabouri M et al., which found no correlation between age and the development of liver fibrosis [32]. Serum ferritin levels were higher in patients with fibrosis, as assessed by Fibroscan, showing a significant positive correlation with the degree of fibrosis, consistent with the findings of Mirault T et al., [33].
Serological tests, such as APRI and FIB-4, are commonly used to suggest fibrosis and can be easily calculated from routine laboratory tests. These indices have been employed with varying success to assess liver fibrosis and cirrhosis in conditions like NAFLD, HCV, and other chronic liver diseases [14]. However, limited studies have evaluated their role in liver dysfunction due to iron overload in young patients with Thalassaemia major. A previous study by Yosef AL et al., found statistically significant positive correlations between LSN values from Fibroscan and other fibrosis markers (APRI, FIB-4, AST/ALT) in thalassaemic children with HCV [34]. In the present study, both APRI and FIB-4 showed excellent diagnostic performance in predicting fibrosis, with high AUROC values. The AUROC for APRI was 0.937 (95% CI: 0.84–1), indicating very strong predictive capability and statistical significance (p<0.001). At a cut-off of APRI ≥0.356, the sensitivity was 85%, and the specificity was 98%, highlighting its high accuracy for fibrosis detection. Similarly, the AUROC for FIB-4 was 0.926 (95% CI: 0.846-1), also demonstrating strong predictive ability (p<0.001). With a FIB-4 cut-off of ≥0.879, the sensitivity was 77%, and the specificity was 97%. These results suggest that both indices are highly reliable, with APRI having a slightly higher sensitivity, while both maintain very high specificity, making them valuable non invasive tools for identifying fibrosis. Both parameters showed a significant positive correlation with YKL-40 as well, with statistically significant results.
YKL-40 is emerging as a new biomarker of inflammation that acts through different pathways compared to routine serum inflammation markers [35]. Studies have demonstrated that YKL-40 levels correlate positively with the severity of liver fibrosis, suggesting its potential as a biomarker for monitoring hepatic complications in thalassaemia patients [9,36]. Elevated YKL-40 concentrations may indicate an inflammatory response in the liver, as chronic inflammation is known to contribute to the progression of fibrosis and cirrhosis [37].
The median YKL-40 level in the current study was 300 (160-735) pg/mL, consistent with findings from Musumeci M et al., where serum YKL-40 concentrations were reported to be within the normal range [36]. However, this was in stark contrast to a study conducted by El-Asrar MA et al., where the mean YKL-40 level in children and adolescents with thalassaemia major was 900 pg/mL [9]. This discrepancy may be attributed to effective adherence to iron chelation therapy and appropriate transfusion protocols, both of which help reduce intestinal iron absorption.
The present study revealed a significant positive correlation between YKL-40 values and liver stiffness as measured by TE, and this correlation was statistically significant. This finding is consistent with studies conducted by El-Asrar MA et al., and Rath T et al., which showed that serum YKL-40 values correlated with histologic liver staging and stiffness as determined by TE [9,38]. Although both are non invasive tests, YKL-40 may be preferred over TE as it is a blood biomarker, eliminating the need for the specialised FibroScan device. This makes it more accessible in settings where FibroScan is unavailable. TE requires trained doctors or technicians for accurate execution, whereas measuring YKL-40 can be easily performed using an ELISA, as demonstrated in the present study. FibroScan devices are expensive, making them inaccessible for many Institutions. Additionally, their accuracy diminishes in patients who are obese, have ascites, or suffer from liver inflammation. In contrast, YKL-40 is a serum biomarker unaffected by these physical factors. It can be seamlessly integrated into routine laboratory assessments, particularly during regular follow-ups for transfusion-dependent patients.
In the current study, the median YKL-40 levels (pg/mL) were found to be highest in the group with ferritin levels exceeding 5000 ng/mL. This finding aligns with the study by El-Asrar MA et al., which reported a significant elevation of YKL-40 in patients with higher ferritin levels, indicating a correlation with iron overload [9].
In an earlier study by Tran A et al., YKL-40 levels were measured in 146 consecutive heavy drinkers (106 men and 40 women; mean age 49.2±9.0 years). The results revealed that plasma YKL-40 levels increased in correlation with the severity of fibrosis (p<0.00001), highlighting its potential role as a biomarker for assessing liver fibrosis [39].
In a study conducted by El-Asrar MA et al., 50 children and adolescents with beta-thalassaemia major were compared to 35 healthy controls to investigate the correlation between serum YKL-40 levels and liver fibrosis in thalassaemia patients. The findings revealed that YKL-40 levels positively correlated with several parameters, including the transfusion index (r=0.428, p=0.008), alanine aminotransferase (r=0.368, p=0.008), lactate dehydrogenase (r=0.878, p<0.001), serum ferritin (r=0.721, p<0.001), and liver iron concentrations. Conversely, YKL-40 levels were negatively correlated with MRI-T2* values. These correlations suggest that YKL-40 could serve as a valuable adjunctive tool for estimating liver fibrosis in patients with beta-thalassaemia major [9].
In another study by Rath T et al., 55 patients with chronic liver disease underwent TE, and serum YKL-40 concentrations were measured using ELISA. YKL-40 demonstrated the highest diagnostic accuracies among serum markers, with values of 0.792 for F>2 and 0.914 for F>3. The findings indicate that YKL-40 is a potent fibrosis marker, particularly for Hepatitis C Virus (HCV)-associated liver disease, and may enhance the diagnostic accuracy of TE in the early stages of fibrosis [38].
In a study by Yan L et al., serum YKL-40 levels rose significantly with the progression of fibrosis, especially in patients ALT levels below twice the upper limit of normal (p<0.0001). Multivariate analysis indicated that serum YKL-40 was an independent marker of significant fibrosis, confirming its potential as a reliable biomarker for liver fibrosis in this patient cohort [21].
The AUROC for YKL-40 (pg/mL) in predicting fibrosis was 0.971 (95% CI: 0.924-1), indicating outstanding diagnostic performance. This result was statistically significant (p<0.001). At a cutoff of YKL-40 (pg/mL) ≥2100, the test demonstrated a sensitivity of 92% and a specificity of 97% in predicting fibrosis. The high AUROC underscores the potential of YKL-40 as a strong diagnostic marker for fibrosis. These findings suggest that YKL-40, at an optimal cutoff, could serve as a reliable non invasive tool for early fibrosis detection, aiding in timely intervention and management in at-risk patients. Both APRI and FIB-4 index showed a significant positive correlation with YKL-40, further supporting the effectiveness of YKL-40 as a reliable biomarker for assessing liver fibrosis.
Limitation(s)
There are certain limitations to present study. There is a lack of previous validation for APRI and FIB-4 cut-offs predicting fibrosis in younger patients with thalassaemia major, along with the undetermined significance of intermediate values of these scores. The study relies solely on non invasive methods for comparisons, without a definitive comparison to liver biopsy. Additionally, the number of study subjects was small, and there was no follow-up.
Conclusion(s)
The present study underscores the diagnostic potential of non invasive liver parameters, including APRI, FIB-4, and YKL-40, in detecting liver fibrosis in children with beta-thalassaemia major. Among the various non invasive markers, YKL-40 (pg/mL) was the most reliable marker in terms of sensitivity, negative predictive value, and diagnostic accuracy, while APRI exhibited the highest specificity and positive predictive value. Given that hepatic fibrosis significantly contributes to morbidity and impaired quality of life, these non invasive and cost-effective methods present a promising alternative to traditional diagnostics. However, further longitudinal studies are needed to establish optimal cut-offs and validate their long-term effectiveness.