The PCOS is a diverse disease characterised by chronic anovulation and features of hyperandrogenism [1,2], affecting between 9 to 18% of women in the reproductive age group, according to diagnostic criteria [1-3]. Women experiencing PCOS are commonly found to have a Vitamin D deficiency. Lower serum concentrations of 25(OH)D have been observed in 67-85% of women [4]. Symptoms of PCOS have been reported to intensify with Vitamin D deficiency in some observational studies. Furthermore, PCOS has been associated with hirsutism, hyperandrogenism, obesity, elevated cardiovascular disease risk factors, menstrual and ovulatory irregularities, lower pregnancy success rates, and Insulin Resistance (IR) [4].
Several studies with small sample sizes have suggested connections between VDR polymorphisms and the development of PCOS and IR [8-10]. In PCOS, the role of certain variants of genes involved in cholesterol synthesis (DHCR7) and its hydroxylation (CYP2R1), and ultimately Vitamin D synthesis, is still subject to further investigation [7].
With this background, the aim was to study the association of DHCR7 gene polymorphism with metabolic and endocrine parameters, including 25(OH)D levels in women with PCOS. The study also analysed the association of this gene polymorphism with the occurrence of PCOS.
Materials and Methods
The present cross-sectional study was conducted at the Department of Biochemistry, Calcutta National Medical College, Kolkata, West Bengal, India, from January 2018 to February 2020. Due ethical clearance was obtained from the Institutional Ethical Committee, bearing number CNMC/10, prior to the commencement of the study. The protocols of the study were in accordance with the guidelines of the Helsinki Declaration, 1975, revised in 2000.
Inclusion criteria:
Diagnosis of PCOS based on the revised Rotterdam criteria;
Age group: 16 to 45 years.
Exclusion criteria:
Recent pregnancy or lactation.
Conditions related to vitamin D deficiency disorders of the kidney, liver, gastrointestinal system, as well as malnourished cases.
Patients on regular calcium and Vitamin D supplements.
Sample size calculation: The sample size of 79 was determined based on a study by Dipanshu S and Chakravorty R, [11]. The minimum sample size calculated was 79.
Study Procedure
Diagnostic criteria of PCOS: PCOS was diagnosed according to the Rotterdam PCOS consensus criteria (The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2003) [6]. For present study, samples were collected from 100 patients as well as a control group. However, since Deoxyribonucleic Acid (DNA) extraction was performed manually using the phenol-chloroform extraction method and only 3 mL of sample was collected for DNA extraction, authors were able to extract DNA from only 78 cases and 50 controls. Thus, the study consisted of 78 women with PCOS, aged 16-45 years, who were routinely referred to the Gynaecology outpatient clinic for PCOS evaluation, and 50 age-matched women were taken as the control group. All control women had normal thyroid function, regular menstrual cycles, normal serum androgens, and no clinical signs of hyperandrogenism. Informed consent was obtained from all participants, and assent was obtained from the legal guardians of those aged below 18 years.
Parameters: A 10 mL blood sample was taken from an antecubital vein after an 8-12 hour overnight fast, between 8:00 to 10 am. From this sample, 3 mL of blood was collected in an Ethylenediaminetetracetic Acid (EDTA) vial for genomic DNA extraction and PCR analysis, while the rest of the blood was used for the estimation of other parameters. Total Cholesterol (TC), High-density Lipoprotein Cholesterol (HDL), Triglycerides (TG), Low-Density Lipoprotein (LDL), and fasting glucose levels were determined using auto-analysers. Total testosterone, insulin, and serum 25(OH) D levels were measured by Enzyme-linked Immunosorbent Assay (ELISA) (Accubind, USA). The sensitivity and specificity of the ELISA kits were >0.95 in comparison to Chemiluminescence Immunoasssay (CLIA). Anthropometric measurements were obtained from each subject. Waist Circumference (WC) and hip circumference were measured following standard techniques. The Body Mass Index (BMI) was calculated as weight in kilograms divided by the square of height in meters. IR was estimated using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) [12].
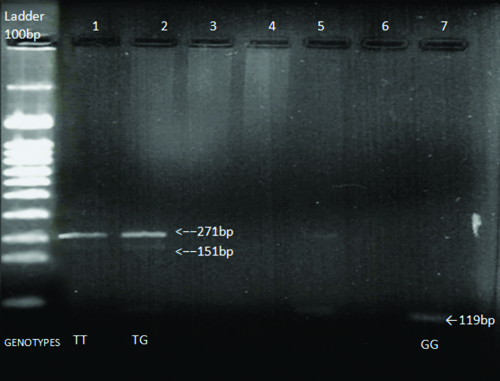
Genotyping: The extraction of genomic DNA was performed using 3 mL of fresh whole blood via the phenol-chloroform extraction method. Molecular genotyping for the DHCR7 (rs12785878) Single Nucleotide Polymorphism (SNPs) was conducted using PCR. Amplification of the DHCR7 gene was carried out using the forward primer 5´-ACC ACC TTC AAA TAG GGC TGT-3´ and the reverse primer 5´-CAG CAG ACA GGA CAT GAG GAT-3´. The reaction conditions were as follows: initial denaturation at 94°C for four minutes, followed by 30 cycles of denaturation at 94°C for one minute, annealing at 56°C for 45 seconds, and polymerisation at 72°C for one minute each; the final extension was carried out at 72°C for five minutes. The analysis of PCR products was performed through electrophoresis in 1.5% agarose gels and visualised under Ultraviolet (UV) light. Only PCR products exhibiting a single amplification without any evidence of non specific amplification were used for DNA sequencing. The PCR products devoid of non specific bands were selected. The obtained PCR product, measuring 271 bp in length, was further digested with the TaqI restriction enzyme according to the protocol received from the supplier (GeNei). The DHCR7 polymorphism creates a restriction site for TaqI, resulting in the cleavage of the 271 bp product into 151 bp and 119 bp fragments. The digestion products were analysed post-agarose gel electrophoresis and visualised with ethidium bromide for the identification of polymorphism. The protocol for genotypic identification is as follows: a single band of 151 base pair (bp) indicates TT, two bands of 151 bp and 119 bp indicate TG, and a single band of 119 bp indicates GG [Table/Fig-1].
Identification of different genotypes using PCR-RFLP of DHCR7 gene polymorphism in Vitamin D levels associated gene.
Statistical Analysis
The data evaluation was conducted using the Statistical Package for Social Sciences (SPSS) version 20.0. The genotypic frequency of DHCR7 in PCOS cases and healthy controls was calculated using a Z-test. Continuous variable data were expressed as mean±Standard Devaition (SD) and analysed using Analysis of Variance (ANOVA). Within the group of women with PCOS, ANOVA with Post-hoc analyses (Bonferroni test) was employed to compare continuous parameters between genotypes. A p-value of <0.05 was considered statistically significant.
Results
The mean age was 26.73±7.4 years for PCOS subjects and 27.68±9.2 years for healthy controls, with a p-value that was not statistically significant (p=0.571). In present study, the frequency of genotypic distribution of the DHCR7 gene among PCOS women and healthy controls with regular ovulatory cycles was similar and statistically not significant (p=0.165), as represented in [Table/Fig-2].
Shows genotypic frequency of DHCR7 in PCOS cases and healthy controls using.
| DHCR7 | Groups | Total | p-value |
|---|
| Case | Control | |
|---|
| TT | 43 (55.1%) | 28 (56%) | 71 | 0.165 |
| TG | 31 (39.7%) | 15 (30%) | 46 |
| GG | 4 (5.1%) | 7 (14%) | 11 |
| Total (N) | 78 | 50 | 128 | |
Z-test; p-value of <0.05 was considered to be statistically significant
The endocrine parameters, including Follicular Stimulating Hormone (FSH), Luteinising Hormone (LH), LH/FSH, prolactin, and testosterone, were not statistically significant between the individual genotypic variants. Fasting insulin, FBS/insulin ratio, and HOMA-IR did not show any statistical differences between individual genotypes. Overall lipid profiles, lipid ratios, as well as the Triglyceride Index (TyG) index did not show any significant differences between the genotypes. The WHR differed statistically among the individual genotypes (p<0.001). Post-hoc analysis revealed that both TG and TT differed significantly (p<0.001), as well as GG and TT (p=0.003). The TT genotype differed significantly from both the TG and GG genotypes individually [Table/Fig-3].
Comparison of clinical and endocrine parameters between different genotypes of DHCR7 in PCOS patients.
| Parameters | TT | TG | GG | p-value |
|---|
| BMI (kg/m2) | 24.77±5.7 | 21.96±4.27 | 21.15±6.86 | 0.059 |
| WHR | 0.93±0.07 | 0.85±0.036 | 0.82±0.05 | <0.01 |
| FSH (mIU/mL) | 13.15±8.5 | 17.14±14.04 | 13.6±7 7.5 | 0.308 |
| LH (mIU/mL) | 16.9±11.98 | 28.07±27.99 | 14.93±5.38 | 0.053 |
| LH/FSH ratio | 2.07 | 2.78 | 1.16 | 0.56 |
| Prolactin (ng/mL) | 49.36±47.37 | 41.78±37.7 | 24.46±15.2 | 0.47 |
| Testosterone (nmol/L) | 0.75±1.01 | 0.68±0.92 | 0.51±0.27 | 0.878 |
| FBS (mg/dL) | 92.67±20.71 | 82.19±11.9 | 7 9.50±13.68 | 0.029* |
| Fasting insulin (mU/L) | 17.9±27.27 | 12.4±8.39 | 16.08±20.24 | 0.556 |
| FBS/Insulin | 24.58±58.4 | 11.37±10.26 | 12.09±8.56 | 0.431 |
| HOMA-IR | 3.84±4.96 | 2.57±1.77 | 2.71±2.87 | 0.378 |
| TC (mg/dL) | 164.44±47.37 | 158.16±49.29 | 164±56.02 | 0.857 |
| TG (mg/dL) | 124.56±60.28 | 108.45±55.61 | 93.25±43.03 | 0.358 |
| HDL (mg/dL) | 44.35±14.51 | 44.10±16.12 | 47±9.27 | 0.936 |
| LDL (mg/dL) | 81.07±32.79 | 84.23±32.57 | 85.75±50.58 | 0.906 |
| VLDL (mg/dL) | 29.51±17.35 | 23.9±10.83 | 18.75±8.65 | 0.155 |
| TG/HDL | 2.95±1.21 | 2.65±1.17 | 2.014±0.98 | 0.243 |
| TC/HDL | 3.89±0.87 | 3.84±0.98 | 3.42±0.62 | 0.61 |
| TyG | 4.6±0.26 | 4.47±0.26 | 4.4±0.24 | 0.069 |
| VIT D (ng/mL) | 37.84±26.67 | 27.43±21.12 | 52.2±55.3 | 0.103 |
ANOVA; p<0.05 was considered to be statistically significant; VLDL: Very low density lipoprotein
Fasting Blood Glucose (FBS) among PCOS genotypes was statistically significant (p=0.029). Post-hoc analysis indicated that the mean FBS statistically differed between TT and TG (p=0.038), with the “TT” genotype showing a slightly increased tendency for FBS compared to the “TG” genotype [Table/Fig-4].
Post-hoc analysis (Bonferroni).
| Dependent variables | (I) DHCR7 | (J) DHCR7 | Mean difference (I-J) | Std. error | Sig. |
|---|
| BMI | TT | TG | 2.811 | 1.249 | 0.082 |
| GG | 3.621 | 2.745 | 0.573 |
| TG | TT | -2.811 | 1.249 | 0.082 |
| GG | 0.810 | 2.795 | 1.000 |
| GG | TT | -3.621 | 2.745 | 0.573 |
| TG | -0.810 | 2.795 | 1.000 |
| W/H | TT | TG | 0.0786909* | 0.0134799 | <0.001 |
| GG | 0.1034651* | 0.0299068 | 0.003 |
| TG | TT | -0.0786909* | 0.0134799 | <0.001 |
| GG | 0.0247742 | 0.0303954 | 1.000 |
| GG | TT | -0.1034651* | 0.0299068 | 0.003 |
| TG | -0.0247742 | 0.0303954 | 1.000 |
| FBS | TT | TG | 10.481 | 4.11 | 0.038 |
| GG | 13.174 | 9.121 | 0.458 |
| TG | TT | -10.481 | 4.11 | 0.038 |
| GG | 2.694 | 9.27 | 1 |
| GG | TT | -13.174 | 9.121 | 0.458 |
| TG | -2.694 | 9.27 | 1 |
Although there were no significant differences in BMI among the genotypes (p=0.059), post-hoc analysis revealed significant variations between TT and TG (p<0.001) as well as TT and GG (p=0.003). Again, the “TT” genotype was associated with a higher risk compared to both TG and GG genotypes.
An independent association of the genetic variants of DHCR7 polymorphisms in PCOS was found with respect to FBS (TT >TG >GG), W/H ratio (TT >TG >GG), and BMI (TT >TG and GG), indicating that certain genotypes are more prone to developing IR and MetS. Vitamin D levels for each individual genotype in PCOS women were 37.84±26.67 ng/mL for TT, 27.43±21.12 ng/mL for TG, and 52.2±55.3 ng/mL for GG, respectively (p-value=0.103).
Discussion
In the present study, the frequency of the DHCR7 gene and its genotypes was similar in women with PCOS and healthy controls with regular ovulatory cycles. A significant difference in the W/H ratio between the genotypes was one of the novel findings in this study (p<0.001). The TT genotype differed significantly from both the TG and GG genotypes individually. The mean 25(OH)D levels in PCOS women concerning DHCR7 polymorphism were 37.84 ng/mL for TT, 27.43 ng/mL for TG, and 52.2 ng/mL for GG, respectively, and did not differ significantly (p=0.103).
There is a lot of evidence suggesting a correlation between vitamin D deficiency and the pathogenesis of PCOS [7,13]. Several studies support the role of Vitamin D in the physiological aspects of the female reproductive cycle, extending beyond just VDR [13-15]. The active form of Vitamin D, 1,25(OH)2D, has been shown to control the expression and secretion of hormones, such as human chorionic gonadotropin and human placental lactogen. Another function of active Vitamin D is its regulation of endometrial decidualisation [15]. Additionally, active Vitamin D is responsible for the in-vitro induction of hormones such as progesterone, estradiol, and estrone. Oestrogen in females requires Vitamin D for its proper synthesis [16]. VDR has been detected in the decidua as well as in the placenta [13]. VDR and traces of enzymes that metabolise Vitamin D have been found in syncytiotrophoblasts extracted from human cultures. VDR polymorphisms are thought to contribute to or increase the risk of PCOS. Supporting this theory, 1,25(OH)2D regulates a number of genes, including those related to glucose homeostasis. Studies have associated VDR polymorphisms, such as Apa 1, Taq 1, Cdx2, Bsm 1, and Fok 1, with metabolic pathways in PCOS. The rs757343 single-nucleotide polymorphism has also been linked to the severity of PCOS symptoms, but not to the likelihood of developing the disease [17]. Several studies have implicated the role of vitamin D gene metabolism and its transport in the development of PCOS [7,18,19]. These observations paved the way for designing present study. The study population was kept homogeneous to prevent any ambiguity.
The DHCR7 gene encodes the enzyme 7-dehydrocholesterol (7-DHC) reductase, which converts 7-DHC to cholesterol. 7-DHC reductase removes 7-DHC, a precursor of 25(OH)D, from the synthetic pathway of vitamin D3. In a study by Wehr E et al., an association between DHCR7 polymorphism and 25(OH)D levels, as well as metabolic parameters including glucose, insulin, and lipids, was found. Most of these associations were attenuated in multivariate analyses, indicating that they are most likely mediated through 25(OH)D levels, which underscores the important association of Vitamin D with IR and its action. However, these polymorphisms may be vital in risk prediction or estimation in women with PCOS [7].
In present study, we did not find any statistical difference between the genotypes concerning vitamin D status, which contrasts with the findings of Wehr E et al., [7] who reported statistically significant lower vitamin D levels among the genotypes. Although all anthropometric and metabolic parameters did not differ significantly in this study, a few, including FBS, W/H ratio, and BMI, did show significant differences among the PCOS genotypes. FBS differed significantly among the genotypes (p=0.029), with the TT genotype exhibiting increased levels compared to TG and GG. This finding aligns with the results of Wehr E et al., who also observed significantly different FBS among the genotypes (p=0.036).
The BMI did not differ significantly (p=0.059) among the genotypes; however, post-hoc analysis revealed significant variations between individual genotypes (TT and TG p=0.00; GG and TT p=0.003), implying that the TT genotype might be associated with a higher risk compared to both the TG and GG genotypes. The results are consistent with a study conducted by Georgopoulos NA et al., which found that women with PCOS had a lower basal metabolic rate compared to women without PCOS, although they did not perform any genetic variations [20]. In our study, the mean BMI of the individual genotypes was normal (TT=24.77, TG=21.96, GG=21.55) and did not vary significantly (p=0.059).
Limitation(s)
The drawback of present study is its small sample size; further research on a larger scale is necessary to prove the susceptibility of PCOS due to DHCR7 polymorphism.
Conclusion(s)
There was no statistically significant association found between the DHCR7 gene polymorphism and 25-OHD levels, as well as other parameters in women with PCOS. However, it was observed that the susceptibility of individual genotypes in women with PCOS to alterations in certain parameters was directly related to the aggravation of symptoms and the risk of MetS.
Z-test; p-value of <0.05 was considered to be statistically significant
ANOVA; p<0.05 was considered to be statistically significant; VLDL: Very low density lipoprotein