Daycare surgeries are on the rise, increasing the demand for daycare anaesthesia [1]. Thus, there is a need for an ideal anaesthetic agent characterised by rapid induction, prompt recovery, and minimal side-effects. Propofol has emerged as the most suitable anaesthetic induction agent, gaining popularity due to its rapid onset, rapid elimination, and clear-headed recovery [2]. However, Propofol is not devoid of adverse effects, such as hypotension, bradycardia, anaphylactic reactions, and pain on injection [3]. Hypotension is a significant cause of morbidity during anaesthesia in hypertensive patients [4]. Hypertensive patients have increased vasoreactivity, which helps regulate blood pressure. In hypertensive patients, the baroreflex is reset to a higher level to maintain blood flow to organs at a higher blood pressure, protecting perfusion even during a hypertensive crisis [5]. Despite this protective mechanism, hypertensive patients are less capable of tolerating minimal hypotension, which compromises perfusion. Propofol-induced hypotension is dose-dependent, so decreasing the total dose requirement can reduce associated morbidity [3]. Various methods have been described in the literature to reduce Propofol dose requirements, such as the simultaneous use of nitrous oxide, opioids, and barbiturates [6]. Other methods, like preloading, slow administration of Propofol, parallel use of vasoactive agents, and the priming principle, have also been found to be effective [7-9]. The priming principle involves administering a predetermined sub-anaesthetic dose of the inducing agent prior to the complete dose [8]. Hypertensive patients face a higher risk of hypotension during the induction of general anaesthesia due to the vasodilatory effects of induction agents [10]. Previous researchers have shown that Propofol priming facilitates a gradual induction with a reduced total Propofol dose, thereby minimising the risk of hypotension and maintaining stable haemodynamic parameters during the induction of general anaesthesia [3,6,11-15]. However, the safety of priming techniques during induction in hypertensive patients has not been studied at all. To fill this gap in the literature, the current study aimed to evaluate the effects of priming on Propofol induction in controlled hypertensive patients, with the objective of observing haemodynamic parameters and total Propofol dose requirements.
Materials and Methods
The prospective, randomised, double-blind comparative study was conducted in the Department of Anaesthesiology, Pt. B.D. Sharma PGIMS, Rohtak, Haryana, India, from April 2023 to April 2024. The study complied with the ethical standards of the Institutional Biomedical Research Ethics Committee and received formal approval (no. BREC/22/TH/Anaesth-22). It was also registered with the Clinical Trial Registry of India (registration no. CTRI/2023/03/050586).
Sample size calculation: A total sample size of 60 patients, with 30 patients in each of the two groups, was calculated based on the study by Suganya S et al., [11]. For the sample size calculation, a mean difference of 14.25 was used, with a 95% confidence interval, 80% power, and an alpha level of 0.05.
Inclusion criteria: Patients aged 18 to 60 years, of either sex, with controlled hypertension (blood pressure <140/90 mmHg) who had been on antihypertensive treatment for atleast eight weeks and were scheduled for elective surgery under general anaesthesia were included in the study after obtaining written informed consent.
Exclusion criteria: Patients were excluded if they had a history of allergy to the study drug, an anticipated difficult airway, a Body Mass Index (BMI) over 30 kg/m2, were pregnant, or were unwilling to participate in the study.
Study Procedure
Patients were randomised into two groups using computer-generated randomisation numbers. Group 1 (study group, n=30) received 25% of the calculated dose of 2 mg/kg of Propofol intravenously as a priming dose [11]. Group 2 (control group, n=30) received 5 mL of normal saline intravenously.
The priming drug was prepared in a 5 mL syringe by an anaesthesia technician not involved in the study. Blinding was achieved by wrapping the loaded syringe in opaque paper. Priming and induction were carried out by an anaesthesiologist not involved in the study. All observations and vital signs were recorded by the investigator of the study. All patients were examined the day before surgery during the preoperative visit. They were allowed to consume solids upto six hours and liquids upto two hours prior to surgery.
The anaesthesia procedure was standardised. A peripheral intravenous line was established, and monitoring was conducted, including electrocardiography, pulse oximetry, non invasive blood pressure measurements, and end-tidal carbon dioxide measurements. Patients were preoxygenated for three minutes with 100% oxygen. Prior to induction, premedication was administered with an intravenous injection of glycopyrrolate (0.004 mg/kg) and an intravenous injection of fentanyl (2 mcg/kg).
In Group 1 (study), Propofol was used for priming, with a priming dose set at 0.5 mg/kg, constituting 25% of the calculated induction dose of Propofol (2 mg/kg). Priming commenced 3 minutes following premedication and was administered over 10 seconds [11]. Conversely, in Group 2 (control), 5 mL of saline was administered over the same 10-second duration. After two minutes of the priming dose, patients in both groups were induced with an injection of Propofol, administered at a rate of 30 mg/10 seconds via a titration method until loss of vocalisation. The cumulative Propofol dosage was recorded. Following induction, a muscle relaxant, inj. Vecuronium bromide 0.1 mg/kg, was given as a loading dose to facilitate endotracheal intubation. Patients were intubated with an adequately sized endotracheal tube, and anaesthesia was maintained by intermittent muscle relaxants, oxygen {Fraction of Inspired Oxygen (FiO2 35%)}, nitrous oxide, and sevoflurane (dial concentration adjusted according to an age-related iso-MAC chart). Haemodynamic parameters, including HR, SBP, Diastolic Blood Pressure (DBP), and MAP, were recorded at various time points. Measurements were taken 30 minutes prior to induction in the preoperative room (T1), at baseline upon transfer to the operating table (T2), immediately after premedication administration (T3), immediately after the priming dose (T4), 2 minutes post-priming (T5), immediately after induction (T6), and three minutes post-induction (T7). If side-effects such as pain at the injection site, pruritus, or anaphylaxis were encountered, they were noted and managed with inj. lignocaine 2% i.v. 1 ml, inj. Pheniramine maleate 44.5 mg i.v. over five minutes, and inj. Adrenaline 0.1 mg/kg Subcutaneous (SC), respectively, along with supportive treatment.
Statistical Analysis
The statistical analysis for the study was conducted using IBM SPSS version 25.0 (SPSS Inc., IBM Corporation, NY, USA), with the data compiled and organised in a Microsoft Excel spreadsheet. Descriptive statistics were computed to determine percentages, means, and Standard Deviation (SD) of the collected data. Before proceeding with the statistical analysis, the dataset was subjected to normality checks using the Kolmogorov-Smirnov test. Subsequently, appropriate statistical tests, such as the Mann’s-Whitney U test, Independent t-test, and Chi-square test, were employed for data analysis. The level of significance was set at p≤0.05, ensuring that results with a probability of occurrence less than or equal to 5% were considered statistically significant.
Results
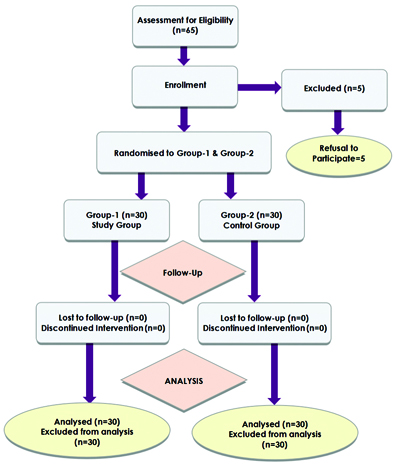
The study initially assessed a total of 65 patients for eligibility. Among these, five patients declined to participate, resulting in 60 patients who were subsequently randomised into two groups: Group 1 (study) and Group 2 (control). The flow of participants through the study process can be visualised in the Consodilated Standards of Reporting Trails (CONSORT) flowchart [Table/Fig-1].
In Group 1 (study), the mean age was 49.36±9.42 years, while in Group 2 (control), it was 49.36±9.19 years (p=0.91). The female-to-male ratio in Group 1 was 23:7, whereas it was 18:12 in Group 2 (p=0.16). The mean BMI was 23.69±3.44 kg/m2 in Group 1 and 24.87±3.56 kg/m2 in Group 2 (p=0.19). These findings suggest that the baseline demographic characteristics, including age, gender distribution, and BMI, were comparable (p>0.05) between the study and control groups, as represented in [Table/Fig-2].
| Demographic parameters | Group 1 (Study) | Group 2 (Control) | p-value |
|---|
| Age (mean±SD) | 49.36±9.42 years | 49.36±9.19 years | 0.901* |
| Gender (n%) | Male | 7 (23.33%) | 12 (40%) | 0.165^ |
| Female | 23 (76.66%) | 18 (60%) |
| Weight (mean±SD) | 66.66±12.28 kg | 62.96±11.85 kg | 0.240* |
| Height (mean±SD) | 163.23±5.39 cm | 162.40±5.46 cm | 0.555* |
| BMI (mean±SD) | 24.87±3.56 kg/m2 | 23.69±3.44 kg/m2 | 0.198* |
*Independent t-test; ^Chi-square test used
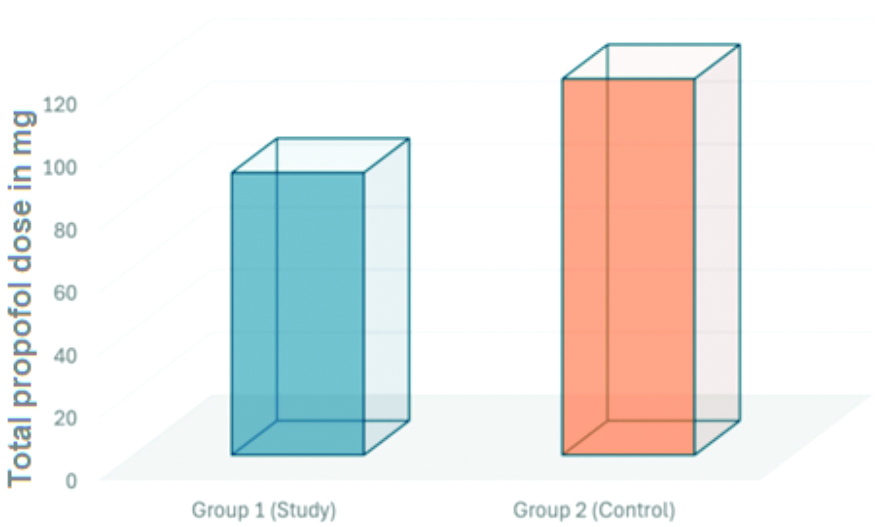
The total dose of Propofol administered differed significantly between the two groups. In Group 1 (study), the mean total Propofol used was 90.07±19.73 mg, whereas in Group 2 (control), it was 120.00±21.81 mg (p=0.001). This indicates a significant difference in the total Propofol dosage between the study and control groups, as represented in [Table/Fig-3].
Total Propofol used among the two groups.
The mean HR per minute at T6 was significantly lower in Group 1 (90.57±11.04) compared to Group 2 (99.60±11.08), with a p-value of 0.002. At the time interval T7, Group 2 had a lower mean HR (78.03±8.90 per minute) compared to Group 1 (84.37±10.50 per minute), with a p-value of 0.015. The mean HRs at different time intervals is depicted in [Table/Fig-4].
Mean Heart Rate (HR) at different time intervals.
| Time interval | Group 1 (Study) | Group 2 (Control) | p-value |
|---|
| 30 min prior to induction in preoperative (T1) | 85.87±13.821/min | 87.70±9.735/min | 0.55 |
| Baseline after shifting on table (T2) | 88.07±14.142/min | 89.00±8.554/min | 0.75 |
| Immediate after premedication (T3) | 84.60±11.467/min | 87.10±8.620/min | 0.34 |
| Immediate after priming dose (T4) | 86.17±10.422/min | 88.10±8.664/min | 0.43 |
| 2 min after priming (T5) | 88.10±10.453/min | 87.07±8.777/min | 0.68 |
| Immediate after induction (T6) | 90.57±11.041/min | 99.60±11.085/min | 0.002(S) |
| 3 min after induction (T7) | 84.37±10.509/min | 78.03±8.908/min | 0.015(S) |
Mann’s-Whitney U test
At T6, the SBP was significantly higher in Group 1 (120.13±5.37 mmHg) compared to Group 2 (95.53±4.60 mmHg), with a p-value of 0.001. Similarly, at the time interval T7, Group 1 exhibited a significantly higher mean SBP (116.20±3.85 mmHg) compared to Group 2 (90.10±4.60 mmHg), with a p-value of 0.001. The SBP at different time intervals is shown in [Table/Fig-5].
Systolic Blood Pressure (SBP) at different time intervals.
| Time interval | Group 1 (Study) | Group 2 (Control) | p-value |
|---|
| 30 min prior to induction in preoperative (T1) | 139.33±2.40 mmHg | 138.80±2.33 mmHg | 0.38 |
| Baseline after shifting on table (T2) | 137.03±7.35 mmHg | 134.40±4.91 mmHg | 0.11 |
| Immediate after premedication (T3) | 131.87±6.53 mmHg | 131.67±8.41 mmHg | 0.91 |
| Immediate after priming dose (T4) | 128.93±6.16 mmHg | 131.03±9.76 mmHg | 0.32 |
| 2 min after priming (T5) | 126.40±5.12 mmHg | 127.57±11.33 mmHg | 0.61 |
| Immediate after induction (T6) | 120.13±5.37 mmHg** | 95.53±5.24 mmHg** | 0.001(S) |
| 3 min after induction (T7) | 116.20±3.85 mmHg** | 90.10±4.60 mmHg** | 0.001(S) |
Mann’s-Whitney U test
At T6, the DBP was significantly higher in Group 1 (78.73±7.11 mmHg) compared to Group 2 (59.87±7.44 mmHg), with a p-value of 0.001. Similarly, at the time interval T7, Group 1 exhibited a significantly higher mean DBP (77.87±5.73 mmHg) compared to Group 2 (56.43±8.47 mmHg), also with a p-value of 0.001. The values at different time intervals are shown in [Table/Fig-6].
Diastolic Blood Pressure (DBP) at different time intervals.
| Time interval | Group 1 (Study) | Group 2 (Control) | p-value |
|---|
| 30 min prior to induction in preoperative (T1) | 86.33±5.84 mmHg | 87.73±3.70 mmHg | 0.27 |
| Baseline after shifting on table (T2) | 86.73±8.64 mmHg | 86.03±5.95 mmHg | 0.71 |
| Immediate after premedication (T3) | 84.97±7.99 mmHg | 82.03±6.45 mmHg | 0.12 |
| Immediate after priming dose (T4) | 79.37±7.72 mmHg | 81.13±5.41 mmHg | 0.30 |
| 2 min after priming (T5) | 79.53±7.60 mmHg | 79.70±4.42 mmHg | 0.91 |
| Immediate after induction (T6) | 78.73±7.11 mmHg** | 59.87±7.44 mmHg** | 0.001(S) |
| 3 min after induction (T7) | 77.87±5.73 mmHg** | 56.43±8.47 mmHg** | 0.001(S) |
Mann’s-Whitney U test
At T6, the mean blood pressure was significantly higher in Group 1 (92.60±5.47 mmHg) compared to Group 2 (71.77±5.28 mmHg), with a p-value of 0.001. Similarly, at the T7 time interval (3 minutes after induction), Group 1 exhibited a significantly higher mean arterial blood pressure (90.63±4.33 mmHg) compared to Group 2 (67.63±5.86 mmHg), with a p-value of 0.001. Mean arterial blood pressure at different time intervals is shown in [Table/Fig-7].
Mean Arterial Pressure (MAP) at different time intervals.
| Time interval | Group 1 (Study) | Group 2 (Control) | p-value |
|---|
| 30 min prior to induction in preoperative (T1) | 104.03±4.05 mmHg | 104.80±2.73 mmHg | 0.39 |
| Baseline after shifting on table (T2) | 103.47±7.26 mmHg | 102.20±4.43 mmHg | 0.41 |
| Immediate after premedication (T3) | 100.67±6.66 mmHg | 98.57±6.27 mmHg | 0.21 |
| Immediate after priming dose (T4) | 95.87±6.07 mmHg | 97.73±5.55 mmHg | 0.21 |
| 2 min after priming (T5) | 94.87±5.65 mmHg | 95.60±5.06 mmHg | 0.59 |
| Immediate after induction (T6) | 92.60±5.47 mmHg** | 71.77±5.28 mmHg** | 0.001(S) |
| 3 min after induction (T7) | 90.63±4.33 mmHg** | 67.63±5.86 mmHg** | 0.001(S) |
Mann’s-Whitney U test
Pain at the injection site was not reported in any patients in Group 1 (study), whereas it was reported in one patient (n=1) in Group 2 (control). Pruritus and anaphylaxis were not reported in either group.
Discussion
In the current study, the Propofol priming principle was assessed in controlled hypertensive patients in terms of total dose, haemodynamic stability, and side-effects. The present study observed a significant decrease in the dose of Propofol when the Propofol priming principle was applied for the induction of general anaesthesia. Consequently, steady haemodynamic parameters were observed compared to the non priming control group. The demographic characteristics, such as age, gender, and BMI, of both groups were found to be comparable, with no significant demographic differences between the study and control groups, indicating a balanced representation of participants across both groups.
In the present study, it was noted that the mean HR at various time points- 30 minutes prior to induction, baseline after shifting to the table, immediately after premedication, and two minutes after priming- did not exhibit significant alterations between both groups. However, there was a notable increase in HR after administering the induction dose of Propofol immediately after induction in the controls, where priming was not used. This increase was significantly higher in the control group immediately after induction (p=0.002) and at three minutes after induction (p=0.01).
The current findings align with those reported by Kumar AA et al., and Dhanapalan SS and Vyas BM [6,12]. Kumar AA et al., observed an increased HR one minute after the administration of the induction dose, which is consistent with the current observation of an immediate rise in HR following Propofol induction [6]. Similarly, Dhanapalan SS et al., reported an increased HR at one and three minutes after induction, with a more pronounced effect observed in the control group compared to the study group [12].
The observed initial rise in HR immediately after administering the induction dose of Propofol, followed by a subsequent decrease at three minutes, can be attributed to the biphasic response associated with Propofol injection. Immediately after induction, there is a decrease in systemic vascular resistance, leading to reflexive increased sympathetic activity. This response is mediated through baroreceptors situated at the carotid sinus and aortic arch, consequently triggering tachycardia. Thereafter, at three minutes after induction, despite the continued decrease in systemic vascular resistance, the HR begins to decrease below baseline levels. This phenomenon occurs due to the resetting of baroreceptor reflexes, where the cardiovascular system adapts to the changes induced by Propofol administration [16].
The SBP exhibited more pronounced decreases in the non priming group compared to the priming group in this study. These findings are similar to the results of Kumar AA et al., who noted that in the control group, SBP dropped more significantly one minute post-induction, immediately after intubation, and five minutes post-intubation compared to the priming group [6]. The mean SBP immediately after induction and at three minutes post-induction with priming exhibited stability when compared to non priming. Significantly, there was an exaggerated drop in SBP without priming compared to with priming, with a p-value of 0.001. The observed fall in blood pressure, particularly in SBP, can indeed be attributed to a decrease in systemic vascular resistance. This mechanism is supported by a study conducted by Pensado A et al., Karlo R et al., also found a significant fall in SBP in the control group. Both studies provide valuable insights into the dose-dependent nature of blood pressure changes induced by Propofol administration [13,17]. In another study conducted by Colson P et al., on hypertensive patients induced with Propofol, it was confirmed that Propofol drastically reduces the response to norepinephrine, angiotensin II, and vasopressin, which is amplified in hypertensive individuals. Therefore, patients on antihypertensive medications should be considered at high risk for haemodynamic instability. The present study population consisted of controlled hypertensive patients, and a similar response was observed [18].
The observed decrease in DBP was significantly less pronounced when the Propofol priming principle was employed, compared to the non priming method, both immediately after induction and three minutes post-induction (p=0.001). Similar results were reported by Kumar AA, who found that DBP in the priming group was higher one minute after induction compared to the control group. Likewise, Malinowska-Zaprzałka M et al., also reported findings consistent with the present study in their investigation of hypertensive patients administered Propofol [6,19]. They observed a notable decrease in DBP three minutes after induction, similar to the current study.
In conjunction with the changes in systolic and DBP, the current study revealed a significantly lesser decline in mean blood pressure immediately post-induction and at three minutes post-induction in the priming group compared to the non priming group, with a p-value of 0.001 at both time points. In a study by Larsen JR et al., on the effects of Propofol on myocardial function, it was found that the decreased mean arterial blood pressure induced by Propofol could be attributed to reduced cardiac filling or the direct negative inotropic effects of the drug.
In the current study, despite the more pronounced haemodynamic effects observed in Group 2 (control) compared to Group 1 (study), it is noteworthy that all patients received concurrent crystalloid infusions and did not require any additional drug interventions to manage their blood pressure [20]. The total drug requirement for Propofol was notably lower when Propofol priming was employed compared to the control group, where priming was not used (p=0.001). This significant reduction in drug usage following priming highlights the effectiveness of this technique in minimising drug requirements compared to the control group. Moreover, this finding helps elucidate the more pronounced haemodynamic side-effects observed in the non priming group, as the side-effects associated with Propofol are known to be dose-dependent.
These results align with those of Kumar AA et al., who reported a 27.48% reduction in the induction dose with the application of priming [6]. They suggested that the reduction in the total Propofol dose after priming could be due to the anxiolytic properties at sub-hypnotic doses. Similarly, Karlo R et al., utilised 25% of the calculated dose for priming in their study and found a 10.23% reduction in the total dose of Propofol in the study group, which was significantly less than in the control group [13]. Hamid AH et al., observed a 25% reduction in the total Propofol dose using priming when compared to controls [14]. Likewise, Gvalani SK et al., and Kataria R et al., also observed a significant reduction in the total Propofol dose compared to controls where the Propofol priming principle was not used [15,21]. In line with these findings, a significant reduction of 24.94% in the total dose of Propofol was observed, reinforcing the efficacy of priming in minimising drug requirements during induction.
Adverse effects, such as pain at the injection site, were absent in the priming group. One patient in the control group experienced pain during induction. The overall lower incidence of pain at the injection site (3.33%) can be attributed to the prior administration of injection fentanyl (2 mcg per kg) and the use of a larger vein. These results were similar to those of the study conducted by Kumar AA et al., which also reported a lesser incidence of pain (3%). Notably, neither pruritus nor anaphylaxis was observed in either group [6].
Limitation(s)
The present study was conducted at a single-centre on controlled hypertensive patients; hence, further trials on a larger population with uncontrolled hypertensive patients are required.
Conclusion(s)
Recent research has demonstrated that the Propofol priming principle, when used during induction with a controlled rate of administration, significantly reduces the total dose requirement of Propofol. Furthermore, it helps to decrease haemodynamic alterations and the complications associated with Propofol injection. This approach effectively mitigates the dramatic effects of hypotension in hypertensive individuals. Therefore, Propofol priming is strongly advised for hypertensive patients during the induction of general anaesthesia.
*Independent t-test; ^Chi-square test used
Mann’s-Whitney U test
Mann’s-Whitney U test
Mann’s-Whitney U test
Mann’s-Whitney U test