Ovarian cancer is a significant global health concern for women. It is the eighth most prevalent malignancy diagnosed globally [1,2]. India has the third-highest incidence of ovarian cancer and gynaecological malignancies [3]. According to The Surveillance, Epidemiology and End Results (SEER) program of the American National Cancer Institute, the lifetime chance of a woman contracting ovarian cancer is one in seventy-eight, while the likelihood of mortality from invasive ovarian cancer is approximately 1 in 108 [4]. American Cancer Society statistics have reported 19,710 new cases and 13,270 mortalities related to ovarian cancer in 2023 [5]. Epithelial tumours emerge from the epithelial cells covering the surface of ovaries that constitute 85-90% all ovarian cancer [6]. Based on heterogeneous histopathological types and genetic mutation of epithelial ovarian cancer, it is classified into two broad categories: Type I and Type II. Type I tumours are less aggressive, with a reported low-grade, harbouring mutation in BRAF (B-Raf), KRAS (Kirsten rat sarcoma viral oncogene homolog) and PTEN (Phosphatase and TENsin homolog) and known as low-grade ovarian carcinoma. These early tumours account for only 10% of deaths [7]. Type II tumours are high-grade ovarian carcinoma. These are more aggressive, spread rapidly and of high-grade intensity.
Approximately 75% of cases of Type II tumours are detected in the advanced stage and account for 90% of deaths [8-10]. Cancer genome-sequencing studies [11-13] in over 96% of HGSOC have identified a p53 gene mutation. Since p53 is highly mutated in Epithelial Ovarian Cancer (EOC), several studies have attempted to explore the potential of developing mutant p53 as a biomarker [14,15]. Nucleotide sequencing is the gold standard for identifying gene mutation but its use in clinical pathology is restricted due to costly, time-consuming, and labour-intensive protocols. Therefore, immunohistochemical detection of p53 gene can be explored as a convenient method to establish p53 as potential ovarian cancer biomarker [16-20]. An advantage is shorter half-life of wild-type p53 makes it undetectable by immunohistochemistry, whereas over-expressed p53 protein can easily be detected, signalling the existence of p53 mutation. Accumulation of p53 protein within tumour cells create a target for IHC [21,22]. The availability of limited data from Asian population and lack of data of correlation of histopathology of ovarian cancer with presence of p53 mutation led the objective of present study. The present study has been carried out to assess the IHC expression of p53 in LGSOC and HGSOC and correlate it with clinical outcomes.
Materials and Methods
The present analytical observational study was conducted in the Department of Genetics, Maharshi Dayanand University, Rohtak, in collaboration with the Department of Pathology, Pandit Bhagwat Dayal Sharma University of Health Sciences, Rohtak, Haryana, India over a period of July 2019 to May 2023. The study was conducted after the approval from the Institutional Human Ethical Research Committee (Ref no-IHEC/19/07).
Inclusion criteria: Cases of serous ovarian cancer, females of any age with reported serous ovarian cancer, only samples with definite histopathological diagnosis were included in the study.
Exclusion criteria: Patients reported to have any other type of cancer, congenital lesions, unusual tumour types and inadequate samples, all other ovarian cancer tumours except LGSOC and HGSOC, cases without available formalin-fixed paraffin-embedded tissue were excluded from the study.
Tissue samples of 37 cases of ovarian carcinoma could be procured. Histological diagnosis revealed 11 cases as low-grade serous carcinomas and 26 high-grade serous ovarian cases. Patient clinical data like type of tumour, stage of tumour and histological grade, were noted from the tumour registries with the help of gynaecological pathologists. Epidemiological data, like age at onset, menopausal status and survival data, were collected from ovarian cancer patients through direct follow-up. All pathological specimens were classified according to the guidelines of the World Health Organisation (WHO) International Classification of ovarian tumours [23]. Stage was classified according to the International Federation of Gynaecology and Obstetrics staging classification, with the help of pathologist. [24].
Immunohistochemical Detection of p53 Mutation
Sections of ovarian cancer tissue fixed in paraffin were collected and placed on slides coated with poly-L-lysine (Sigma Aldrich). The Master Diagnostica™ rabbit anti-human p53 monoclonal antibody (clone SP5) (REF: MAD-000309QD-R-7) was used as the primary antibody for the p53 protein, while the secondary antibody Master Polymer Plus Detection System (peroxidase) from Master Diagnostica (MAD-000237QK-10), which included Diaminobenzidine chromogen.
First, tissue sections were deparaffinised in xylene for 30 minutes. Then, graded alcohol hydration was carried out. The antigen was retrieved using the microwave. To quench endogenous peroxidase activity, hydrogen peroxidase blocking was used. After that, slides were incubated in a humidifying chamber for 40 minutes with a specific primary antibody, following a 20-minute application of the secondary antibody. Master Polymer Plus detecting System, coupled with horseradish peroxidase enzyme, was applied next. Diaminobenzidine (liquid DAB), a chromogenic substrate, was added to all slides, and incubation was utilised. Every incubation was carried out in a simulated damp chamber at ambient temperature. Brown colouration appeared in sections. All of the slides were washed three times after each step using Tris-buffered saline (TBS, 0.1 M, pH 7.4). Lastly, the sections were mounted with D.P.X. after counterstaining with Mayer’s haematoxylin, dehydrating in ascending ethanol concentrations, and rinsing in xylene. All the chemicals and reagents used in the current investigation were of analytical grade and available commercially.
Positive control for every antibody consisted of tissue samples that showed sufficient immunoreactivity. By omitting out the primary antibodies. For assessment of IHC reaction in each case, nuclear p53 protein expression was analysed. The percentages of p53 nuclear staining were estimated as follows: null type or complete absence, focal or wild type (≥1% and <70% of nuclei showed staining), and diffuse type (≥70% of nuclei showed staining) [25].
Statistical Analysis
The statistical analysis was performed with GraphPad Prism version 8.0.2. The Chi-square test and Fisher’s exact test were used to evaluate the differences between the LGSOC and HGSOC group. Overall Survival (OS) was calculated from the moment of diagnosis to the last follow-up or until death for any reason. The Kaplan-Meier method was utilised to estimate OS probability, and the log-rank test was employed to compare the survival curves.
Results
Primary clinical and pathological characteristics of 37 cases of Serous Ovarian Carcinoma (SOC) were evaluated. Among the 37 cases, 26 (70.27%) were classified as HGSOCs, while 11 cases (29.73%) were low-grade serous ovarian carcinomas (LGSOCs) [Table/Fig-1]. All patients were between the age group of 30 and 73 years, with a mean age at diagnosis of 53.16±9.75 years. Clinically, abdominal distension and co-morbidity were common in all HGSOC and LGSOC cases. Among other noted symptoms of HGSOC cases were weight loss in 18 cases (69.23%), ascites in 16 (61.53%), and lower back pain in 16 (61.53%). Pelvic discomfort was a common symptom in LGSOC. Regarding menopausal status, no correlation was found between LGSOC and HGSOC (p-value=0.6).
Clinico-pathological characterisation of High-Grade Serous Ovarian Cancer (HGSOC) and Low-Grade Serous Ovarian Cancer (LGSOC).
| Clinical features | LGSOC N (%) | HGSOC N (%) | p-value |
|---|
| Total patients | 11 (29.73) | 26 (70.27) | - |
| Age (years) | 50.96 (32-65) | 52.64 (30-73) | - |
| Menopausal status | Premenopausal | 3 (27.27) | 9 (34.62) | 0.66 |
| Postmenopausal | 8 (72.73) | 17 (65.38) |
| FIGO staging | I+II | 7 (63.64) | 6 (23.08) | 0.18 |
| III+IV | 4 (36.36) | 20 (76.92) |
| Co-morbidity | 5 | 16 | - |
| Abdominal distension | 8 | 20 | - |
| Residual diseases | No | 8 (72.73) | 11 (42.31) | 0.090 |
| Yes | 3 (27.27) | 15 (57.69) |
| CA- 125 (median value in U/mL) | 27 | 269 | - |
Twenty out of the 26 cases of HGSOC (76.92%) in the current study were diagnosed at an advanced stage (FIGO Stage III and IV), whereas seven out of the eleven cases (63.64%) of LGSOC were diagnosed at an early stage (FIGO Stage I and II). Statistically, there was no discernible correlation between FIGO stages and residual illnesses, nor p53 expression (p=0.18 and p=0.090, respectively) [Table/Fig-1]. When comparing women with HGSOC to those with LGSOC, it was shown that the former had a considerably higher percentage of advanced-stage disease (76.92% vs. 36.36%), higher median CA125 levels (269 vs. 27 U/mL), and a higher percentage of postsurgery residual disease (57.69% vs. 27.27%).
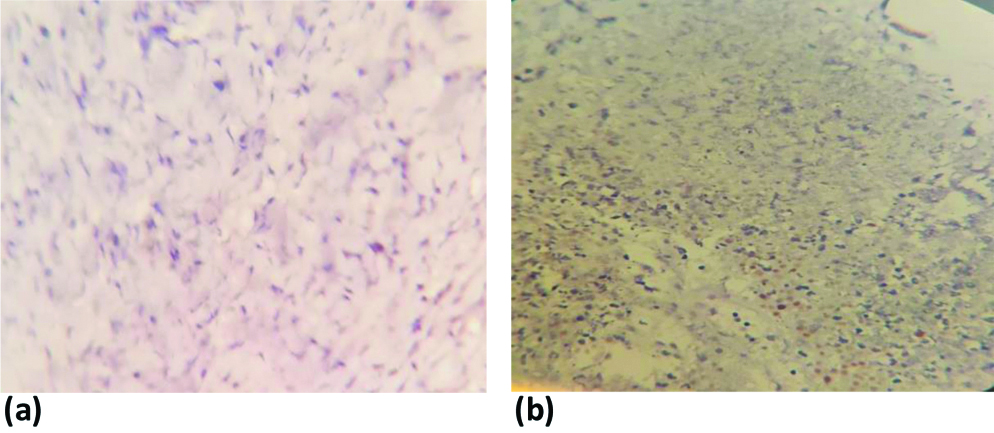
Immunohistochemical evaluation of the 37 cases of serous carcinoma revealed that 17 cases (45.95%) exhibited null p53 expression, 16 cases (43.24%) showed diffuse p53 expression and four cases (10.81%) demonstrated focal positive expression (wild type). The grade of serous ovarian carcinoma and p53 expression showed statistically significant values (p-value=0.02) [Table/Fig-2]. As per histopathological classification, LGSOC exhibited diffuse expression in 9.09%, complete absence in 72.73%, and focal expression in 18.18%, HGSOCs had diffuse expression in 57.69% of cases, null or absent expression in 34.62% (or 92.30% of cases), and focal expression in 7.69% of instances [Table/Fig-3,4].
Immunohistochemical p53 expression in Low-Grade Serous Ovarian Carcinomas (LGSOC) and High-Grade Serous Ovarian Carcinomas (HGSOC).
| IHC expression | LGSOC (N=11) | HGSOC (N=26) | p-value |
|---|
| Focal/wild-type (≥1% and ≤70%) | 2 (18.18) | 2 (7.69) | 0.02 |
| Complete absence/null type | 8 (72.73) | 9 (34.62) |
| Diffuse type (≥70%) | 1 (9.09) | 15 (57.69) |
p-value was calculated using Chi-square/Fisher’s exact test at a significance level of 0.05
p53 staining in HGSOC showing: (a) diffuse; (b) null expression; (c) diffuse; and (d) faint staining expression. All photomicrographs at X200 magnification.

p53 staining in LGSOC showing: (a) null; and (b) focal (wild type) expression. All photomicrographs at X200 magnification.
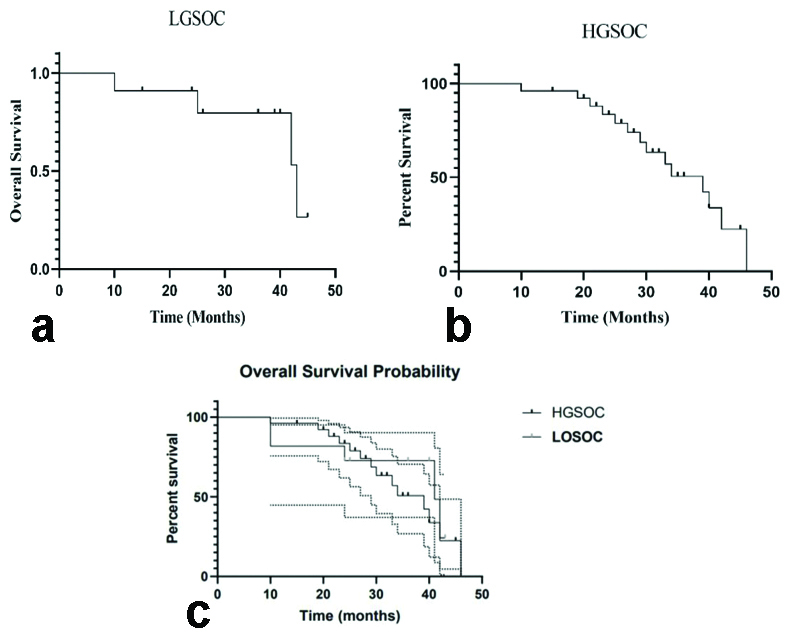
Survival analysis was done for LGSOC and HGSOC. The median follow-up period for the overall group was 36 months (range: 1-46 months). The overall survival (OS) for LGSOC and HGSOC at 40-month follow-up was 72.72% and 33.82%, respectively. Patients with LGSOC had a median OS of 41 months (95% Confidence Interval [CI] 10-43), which was longer than the survival duration for those with HGSOC. The median survival time for high-grade serous ovarian cases was 39 months (95% CI 10-46). Log-rank statistical analysis showed a non significant association between the OS times of the two grades, with a hazard ratio of 1.384 (0.5-3.5) at 95% CI, resulting in a p-value of 0.51 [Table/Fig-5].
a) Overall Survival (OS) in patients with LGSOC; b) Overall Survival (OS) in patients with HGSOC; c): Comparison of Overall Survival (OS) probability curve of patients with LGSOC and HGSOC.
Discussion
Ovarian cancer is a significant global health concern for women. Epithelial tumours emerge from the epithelial cells casing the surface of ovaries that constitute 85-90% all ovarian cancer. Based on heterogeneous histopathological types and genetic mutation of EOC, it can be categorised into two major groups: Type I and Type II. Type I tumours are less aggressive and account for only 10% of deaths [7]. In contrast, Type II tumours are more aggressive, spread rapidly and are of high-grade. Approximately 75% of cases of Type II tumours are detected in the advanced stage and account for 90% of deaths. Cancer genome-sequencing studies in over 96% of HGSOC cases have identified a mutation in the p53 gene [11-13]. IHC expression of p53 was investigated in 37 cases of serous ovarian carcinoma. Out of the total of 37 cases of serous carcinoma, 26 cases were HGSOCs (70.27%) and 11 cases were LGSOCs (29.72%). All the cases were in the age group of 30 to 73 years. The mean age for the LGSOC and HGSOC cases was 50.96 and 52.64 years, respectively. The presence of LGSOC at younger age signifies the increase in the incidence of serous ovarian carcinoma cases with age [26-28].
Statistically significant differences (p-value=0.02) between the grade of serous ovarian cancer and the expression of p53 were observed in the present study. According to reports from Orachum P et al., Yemelyanova A et al., and Na K et al., 93.6-96.75% of HGSOC patients had a p53 immunostaining mutation pattern. In some studies, diffuse type and null type p53 immunohistochemical staining distribution patterns were observed as 60% and 39%, respectively [28-32]. Whereas present study reported a p53 immunostaining mutation pattern in 92.30% of cases of HGSOCs.
Patients with LGSOC have comparable clinical presentations to those with HGSOC. Clinically, all HGSOC and LGSOC cases were reported with co-morbidities and abdominal pain. Among other symptoms of HGSOC, weight loss was reported in 18 cases (69.23%), ascites in 16 (61.53%), and lower back pain in 16 (61.53%). Most LGSOC cases 8 (72.72%) in the present study experienced pelvic discomfort. A total of 28 patients (75.65%) reported abdominal distension, and 21 patients (56.75%) reported co-morbidities. Similar reports are also available in the literature [27,33,34].
In the present study, 20 out of the 26 cases of HGSOC (76.92%) were diagnosed at an advanced stage (FIGO Stage III and IV), whereas seven out of the eleven cases (63.64%) of LGSOC were diagnosed at a relatively early stage (FIGO I and II). Comparing women with HGSOC to those with LGSOC, it was found that the former had a higher percentage of advanced-stage disease (76.92% vs. 36.36%), higher median CA125 levels (median 269 U/mL vs. 27 U/mL), and a higher rate of postsurgery residual disease (57.69% vs. 27.27%). A higher percentage of advanced-stage disease has been reported in HGSOC in the literature [27,32,35].
The majority of serous ovarian cancer cases, 25 (67.56%) in present study, occurred after menopause. However, no significant association was observed between menopausal status and grades of ovarian cancer (p-value=0.66). A statistically non significant association was obtained at p-value <0.05, synchronous with earlier reports (p-value 0.59 and 0.6, respectively) [36,37].
As per available literature, studies have shown better survival patterns for LGSOC have been shown as compared to HGSOC [32,38]. At 40 months of follow-up, the overall survival (OS) for LGSOC and HGSOC was 72.72% and 33.82%, respectively, in the present study. The median OS for patients with LGSOC was 41 months (95% CI 10-43), which was longer than the survival time for those with HGSOC. The median survival time for HGSOC was 39 months (95% CI 10-46). On the survival analysis, unconclusive reports are available in the literature [8,27,28,32,38].
Present study demonstrates distinct p53 expression profiles between two histological subtypes, closely associated with various clinicopathological features. In HGSOC, aberrant p53 expression, characterised by either overexpression or complete absence, strongly correlated with advanced stage at diagnosis, higher tumour grade, increased proliferation indices, and poorer OS. Conversely, LGSOC cases predominantly exhibited wild-type p53 expression, which was associated with earlier stage at diagnosis, lower grade, and better prognosis. Future studies could be focused on and exploring p53-targeted therapies based on specific expression patterns and associated clinicopathological characteristics. By understanding genetic predisposition of ovarian tumour, preventive steps could be undertaken. Immunohistochemical evaluation and clinicopathological correlation can predict involvement of mutation of p53 gene, thereby helping the clinician to plan the early treatment and reduce the severity of fatal disease.
Limitation(s)
Small sample size was the limitation of the present study.
Conclusion(s)
In HGSOC, aberrant p53 expression, characterised by either overexpression or complete absence, strongly correlated with advanced stage at diagnosis and higher tumour grade. It can be hallmark, guiding the diagnosis and indicating the need for aggressive treatment modalities, including chemotherapy and targeted therapies. IHC detection may be explored as a convenient method to establish p53 as potential biomarker along with CA125 to address the severity of ovarian carcinoma. Early diagnosis can pave the way to clinician for planning more personalised and effective treatment strategies in ovarian cancer management.
Authors’ contribution: GB: Data curation, investigation, methodology, visualisation, writing original draft; MV: Conceptualisation, formal analysis, funding acquisition, supervision, validation, visualisation; SS: Patient data curation; GR: Data curation, software handling; NP: Writing and editing.
p-value was calculated using Chi-square/Fisher’s exact test at a significance level of 0.05