The probability of simultaneous Breast Carcinoma (BC) and Endometrial Carcinoma (EC) in one person is extremely low and might be only a co-incidence. Hereby, the authors present a case report of a 62-year-old female presented to the Outpatient Department (OPD) with complaints of postmenopausal bleeding for a period of six months. On examination, her abdomen was enlarged, and a right breast mass was also noted incidentally. An ultrasound of the abdomen showed a thickened endometrium. A screening mammogram revealed a Breast Imaging-Reporting and Data System (BI-RADS) 4B lesion in the lower central quadrant of the right breast. Subsequently, an endometrial aspiration was done and showed features of atypical endometrial hyperplasia. A Tru-cut biopsy of the right breast mass was taken, showed features of a mucinous neoplasm favouring mucinous carcinoma, Nottingham’s histological grade 1. Immunohistochemistry (IHC) work-up favoured a Luminal A molecular subtype of mammary carcinoma. Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET) imaging were also done which revealed a thickened endometrium with increased metabolic activity. Patient underwent a right Modified Radical Mastectomy (MRM), total abdominal hysterectomy with bilateral salpingo-oophorectomy, and lymph node sampling. Grossly, the MRM specimen showed a single, grey-white, firm, infiltrative lesion measuring 2.2×2.2×1.5 cm in the lower central quadrant. Microscopy showed a neoplastic tissue composed of nests of tumour cells floating in lakes of abundant extracellular mucin. The hysterectomy specimen revealed a grey-white to grey-brown endometrial polyp measuring 3.5×2.5 cm, which microscopically showed tumour cells arranged in sheets and in a glandular and papillary pattern with fibrovascular core. IHC for microsatellite instability and p53 were done.
Microsatellite instability, Mucinous neoplasm, Papillary
Case Report
A 62-year-old female presented to the OPD with complaints of postmenopausal bleeding for a period of six months. On complete examination, the patient was found to have an enlarged abdomen and a palpable lump in the right breast. As part of evaluation of the enlarged abdomen, an ultrasound abdomen was done which revealed a thickened endometrium (thickness: 15 mm). An endometrial aspirate was also done, which showed features of atypical endometrial hyperplasia. A mammogram was performed to evaluate the lump in breast, and it revealed a BI-RADS 4B lesion in the lower central quadrant of the right breast, which was subsequently biopsied by Tru-cut needle.
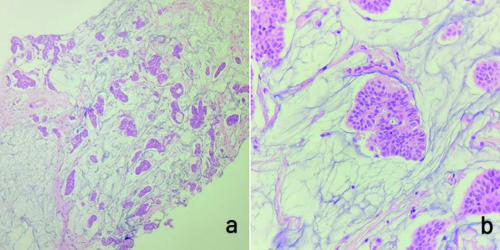
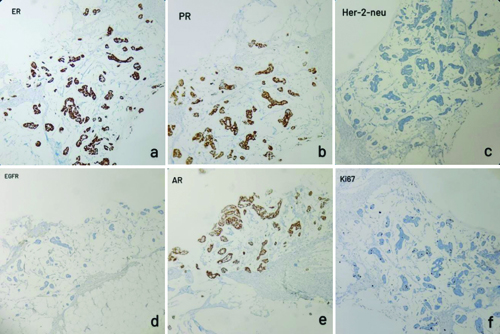
Histopathological {Haematoxylin and Eosin (H&E)} examination of the breast core biopsy showed nests of tumour cells floating in pools of abundant extracellular mucin, and a foci of invasion. The tumour cells had moderate eosinophilic cytoplasm with bland, uniform nuclei [Table/Fig-1]. By IHC the tumour cells were positive for Estrogen Receptor (ER), Progesterone Receptor (PR), and Androgen Receptor (AR), and negative for HER2/neu Epidermal Growth Factor Receptor (EGFR). Ki67 labelling index showed 5% positivity [Table/Fig-2]. These histological features were suggestive of a mucinous carcinoma of breast, and the IHC features suggested of Luminal A molecular subtype of mammary carcinoma.
a) Histology showing a linear core of breast tissue with nests and glands of tumour cells floating in pools of abundant mucin (H&E, 100x); b) High power magnification of the section showing a tumour cell nest with individual cells with moderate eosinophilic cytoplasm and moderately pleomorphic nucleus (H&E, 400x).
IHC staining of the breast Tru-cut biopsy: a,b,e) Tumour cells are positive for ER, PR and AR respectively; c,d) Tumour cells are negative for Her-2-neu and EGFR; f) 5% of tumour cells positive for Ki67 (IHC, 100x).
On further investigation with an MRI pelvis revealed increased thickness (15 mm) of the endometrium, which also showed increased metabolic activity (SUV max: 14.11) in PET imaging, with no metabolically active locoregional lymph nodes. The PET imaging also showed a metabolically active soft tissue density lesion with spiculated margins in the lower aspect of the right breast, measuring 2.1×2.0×1.3 cm, corresponding to the BI-RADS 4B lesion identified in the mammogram.
Based on these radiological findings, the endometrial aspirate showing atypical endometrial hyperplasia, and the breast Tru-cut showing mucinous carcinoma, management of the patient with Modified Radical Mastectomy (MRM) and total abdominal hysterectomy with bilateral salpingo-oophorectomy and lymph node sampling was planned. The patient underwent the same. The MRM specimen measured 32×24×4 cm, which showing a single, grey-white, firm, infiltrative lesion measuring 2.2×2.2×1.5 cm in the lower central quadrant, with no secondary features like necrosis or haemorrhage. The rest of the breast had elastosis.
Microscopy of the lesion from the MRM specimen showed an infiltrative lesion composed of tumour cells arranged in nests, glands, and cords floating in abundant extracellular mucin. The individual cells showed scant to moderate eosinophilic cytoplasm, increased nucleocytoplasmic ratio, and moderately pleomorphic nuclei, some of them showing prominent nucleoli [Table/Fig-3]. The final diagnosis was given as mucinous carcinoma of breast.
a,b) Showing tumour cells forming nests, cords and glands floating in pools of abundant mucin (H&E, 40x, 100x, respectively); c) Showing a nest of tumour cells which have scant to moderate eosinophilic cytoplasm, increased nucleocytoplasmic ratio and moderately pleomorphic nuclei with a few showing prominent nucleoli (H&E, 400x).
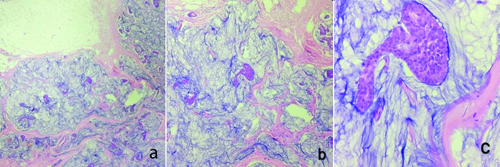
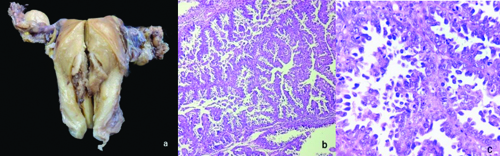
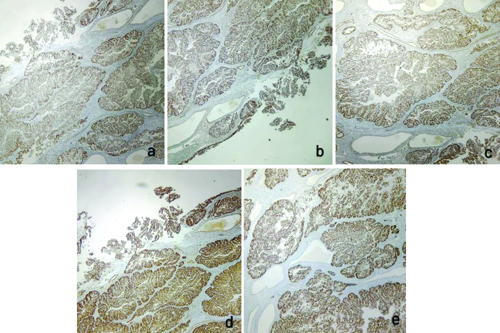
The total abdominal hysterectomy and bilateral salpingo-oophorectomy specimen showed a grey-white to grey-brown endometrial polyp measuring 3.5×2.5 cm, which was soft to firm in consistency. Grossly, the tumour did not seem to invade the myometrium or the lower uterine segment. Microscopy of the polyp showed tumour cells in glandular and tubular architecture, with some showing papillary architecture and slit-like spaces. Some of the glands showed cribriforming. The individual tumour cells had scant to moderate eosinophilic cytoplasm and pleomorphic, hyperchromatic nuclei with prominent nucleoli. An increase in mitotic figures and some atypical mitoses were also noted. No nodal involvement noted microscopically [Table/Fig-4]. By IHC, intact nuclear expression was seen in MLH1, MSH2, MSH6, and PMS2 staining, while p53 staining was strongly and diffusely positive in the tumour cells [Table/Fig-5]. The final diagnosis was established as Mismatch Repair (MMR) proficient, serous endometrial carcinoma (EC). The patient is currently under follow-up after three cycles of chemotherapy and is doing well.
a) Total abdominal hysterectomy with bilateral salpingo-oophorectomy specimen showing a grey white to grey brown, firm, endometrial polyp measuring 3.5×2.5 cm; b) Histology of the polyp showing glands and tubules formed by the tumour cells (H&E, 100x); c) In high power, tumour cells with moderate eosinophilic cytoplasm with pleomorphic nuclei and prominent nucleoli can be noted. Increase in mitotic figures and few atypical mitotic figures can also be noted (H&E, 400x).
a) MLH1; b) MSH2; c) MSH6; d) PMS2. All showing intact nuclear expression; e) p53 showing strong and diffuse positivity. (IHC 100x).
Discussion
Multiple Primary Cancers (MPCs) are defined as two or more new primary independent tumours that develop at the same time or consecutively, from the same or different organ. They must not be metastatic, and the different origins should be verified by IHC examination. The time requirement allows MPCs to be classified as either synchronous (new malignancies appearing within six months) or metachronous (new malignancies appearing beyond six months) [1,2]. The development and pathophysiology of MPCs are influenced by specific processes, but an important role played by genes, particularly BRCA1 and BRCA2. These genes are linked to an increased risk of breast, ovarian, stomach, colon, pancreatic, and uterine cancers [3]. It is relatively rare to diagnose synchronous primary cancers in one patient [4]. The probability of simultaneous Breast Carcinoma (BC) and EC in one person is extremely low and might be only a co-incidence. According to one study, the diagnosis of EC within one year after the diagnosis of a primary BC is less than 0.05%.
The risk factors for EC are age (i.e., it is more common in older patients), nulliparity, and the postmenopausal period [5,6], and a history of irregular menstrual cycles [7]. The hormonal status of the patient also plays a significant role in the development of EC. Reduced oestrogen and increased progesterone exposure can decrease the risk of EC [8]. Obesity, nulliparity, and irregular menstrual cycles may amount to increased oestrogen exposure, so they may contribute to the development of EC. EC may also develop in association with tamoxifen treatment, usually given for BC, especially with long-term usage and high cumulative doses [9-11]. The present patient had risk factors associated with being older and postmenopausal. The patient had not undergone any neoadjuvant chemotherapy. The Breast Cancer (BC) was found in the patient at the same time as the Endometrial Cancer (EC).
Many risk factors can increase the possibility of developing BC, including age, gene mutations, prolonged exposure to oestrogen, family history, and an unhealthy lifestyle [12]. The source of the oestrogen can be exo or endogenous, which increases the risk of BC. Prolonged exposure to oestrogen can be due to menarche at a younger age, late menopause, first pregnancy in a relatively older age. Oophorectomy can reduce the risk of BC in patients with endogenous oestrogen production [13]. The main sources of exogenous oestrogen are Oral Contraceptives (OCPs) and Hormone Replacement Therapy (HRT). Lifestyles involving excessive alcohol consumption and high-fat diets can also increase the risk of BC [14]. Mutations and abnormal amplification of oncogenes like HER2, and antioncogenes such as BRCA1/2, play vital roles in the genesis and progression.
Many environmental and hormonal risk factors may predispose the patient to both BC and EC [15]. In addition to many similar environmental and hormonal risk factors, the same embryological origin of the endometrium and breast itself can be one of the factors that raise the risk of a simultaneous or synchronous tumour in these organs [16,17]. There might be genetic and epigenetic changes, and other plausible molecular mechanisms, that might give rise to synchronous double cancers [18].
Multiple Primary Cancers (MPCs) can be categorised into three groups based on the aetiological factor. They are treatment-related neoplasms, syndromic cases (like Cowden syndrome), and neoplasms that may have common aetiological factors like genetic predisposition or environmental influences [19]. A few published cases have been summarised in [Table/Fig-6] [20-22].
Review of literature of similar synchronous tumour case reports [20-22].
| Reference | Patient details | Microscopy and ancillary studies | Treatment |
|---|
| Banimostafavi ES et al., 2017 [20] | 53/F, mass in supraclavicular region | Breast core - Invasive ductal carcinoma (grade II). IHC: ER, PR, Her-2-neu - Positive, and Ki67- 10% | Post-surgical chemotherapy. |
| Endometrium (1 month later), Endometrioid Endometrial Carcinoma (EC) (stage IIIB, grade II) |
| Cetinkaya K and Yuce E, 2019 [21] | 63/F, mammogram- nodularity in left breast | Breast: Fine Needle Aspiration (FNA) biopsy - suspicious malignancy.Tru-Cut biopsy- invasive Breast Carcinoma (BC).IHC: ER, PR - positive. Cerb-B2- 0, and Ki-67 30%. | Breast conservative surgery and radiotherapy |
| Endometrium-non - endometrioid serous carcinoma type II.Ovary - Primary ovarian granulosa cell tumour. | Chemotherapy and radiotherapy |
| Gaube A et al., 2022 [22] | 69/F, postmenopausal bleeding | Endometrium: Serous Endometrial Carcinoma (EC); p15, p16 proteins - positive, ER - 5-10%, PR negative. | Adjuvant chemotherapy |
| Breast: Core - Invasive ductal carcinoma, NST. ER, PR- positive, Her-2-neu- negative, Ki67- 5-10%.Breast MRM- Lobular carcinoma, Grade 2, score 6 | Postsurgical hormone therapy |
| Present case | 62/F, postmenopausal bleeding. | Breast: Core - Mucinous carcinoma (grade 1)IHC: ER, PR, AR- positive.Her-2-neu, EGFR- negative.Ki67- 5%. | Breast modified radical mastectomy surgery |
| Endometrium - High grade serous carcinoma with papillary features, stage IA.IHC- intact nuclear expression of MLH1, MSH2, MSH6, PMS2. p53- Diffuse and strong positive. | Total abdominal hysterectomy, Bilateral salpingo-oophorectomy and lymph node sampling. |
Conclusion(s)
Tumours of BC and EC is rare to occurs simultaneously. Histopathological and immunohistochemical examination, as stated above, can help in easy differentiation of multiple primaries and metastatic cancers when there are tumours identified at the same time. Many associated risk factors, whether in isolation or as part of MPCs, have been discussed above. In present patient, advanced age and postmenopausal status were identified as associated risk factors.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 14, 2024
Manual Googling: Sep 25, 2024
iThenticate Software: Oct 29, 2024 (14%)
[1]. Romaszko-Wojtowicz A, Buciñski A, Doboszyñska A, Impact of smoking on multiple primary cancers survival: A retrospective analysisClinical and Experimental Medicine 2018 18(3):391-97.10.1007/s10238-018-0498-129550986 [Google Scholar] [CrossRef] [PubMed]
[2]. Jenny N, Toshima ZP, Khalil H, Elisabeth KS, Zakaria E, Per K, Previously diagnosed multiple primary malignancies in patients with breast carcinoma in Western Sweden between 2007 and 2018Breast Cancer Res Treat 2020 184(1):22122810.1007/s10549-020-05822-z32740808 [Google Scholar] [CrossRef] [PubMed]
[3]. Lv M, Zhang X, Shen Y, Wang F, Yang J, Chen Z, Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumoursMedicine (Baltimore) 2017 96(17):e679910.1097/MD.000000000000679928445321 [Google Scholar] [CrossRef] [PubMed]
[4]. Chang-Ching Y, Peng-Hui W, Chiung-Ru L, Min-Shyen Y, Kuan-Cjong CF, Synchronous breast invasive ductal carcinoma and endometrial endometrioid adenocarcinoma: Case reportJournal of Obstetrics and Gynaecology Research- Wiley Online Library 2011 37(9):1246-49.10.1111/j.1447-0756.2010.01502.x21518130 [Google Scholar] [CrossRef] [PubMed]
[5]. Saadat M, Truong PT, Kader HA, Speers CH, Berthelet E, McMurtrie E, Outcomes in patients with primary breast cancer and a subsequent diagnosis of endometrial cancer: Comparison of cohorts treated with and without tamoxifenCancer 2007 110(1):31-37.10.1002/cncr.2273417510927 [Google Scholar] [CrossRef] [PubMed]
[6]. Burke TW, Fowler WC Jr, Morrow CP, Clinical aspects of risk in women with endometrial carcinomaJ Cell Biochem Suppl 1995 23:131-36.10.1002/jcb.2405909178747387 [Google Scholar] [CrossRef] [PubMed]
[7]. Soliman PT, Oh JC, Schmeler KM, Sun CC, Slomovitz BM, Gershenson DM, Risk factors for young premenopausal women with endometrial cancerObstet Gynecol 2005 105(3):575-80.10.1097/01.AOG.0000154151.14516.f715738027 [Google Scholar] [CrossRef] [PubMed]
[8]. Dossus L, Allen N, Kaaks R, Bakken K, Lund E, Tjonneland A, Reproductive risk factors and endometrial cancer: The European Prospective Investigation into cancer and nutritionInt J Cancer 2010 127(2):442-51.10.1002/ijc.2505019924816 [Google Scholar] [CrossRef] [PubMed]
[9]. Van Leeuwen FE, Benraadt J, Coebergh JW, Kiemeney LA, Gimbrñre CH, Otter R, Risk of endometrial cancer after tamoxifen treatment of breast cancerLancet 1994 343(8895):448-52.10.1016/S0140-6736(94)92692-17905955 [Google Scholar] [CrossRef] [PubMed]
[10]. Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE, Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of Liver and Endometrial cancer Risk following TamoxifenLancet 2000 356(9233):881-87.10.1016/S0140-6736(00)02677-511036892 [Google Scholar] [CrossRef] [PubMed]
[11]. Wang PH, Chao HT, A reconsideration of tamoxifen use for breast cancerTaiwan J Obstet Gynecol 2007 46(2):93-5.10.1016/S1028-4559(07)60001-117638615 [Google Scholar] [CrossRef] [PubMed]
[12]. Majeed W, Aslam B, Javed I, Khaliq T, Muhammad F, Ali A, Breast cancer: Breast cancer: Major risk factors and recent developments in treatmentAPJCP 2014 15:3353-58.10.7314/APJCP.2014.15.8.335324870721 [Google Scholar] [CrossRef] [PubMed]
[13]. Endogenous Hormones and Breast Cancer Collaborative GroupSex hormones and breast cancer risk in premenopausal women: Collaborative reanalysis of seven prospective studiesLancet Oncol 2013 14:1009-19.10.1016/S1470-2045(13)70301-223890780 [Google Scholar] [CrossRef] [PubMed]
[14]. Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, Risk factors and preventions of breast cancerInt J Biol Sci 2017 13(11):1387-97.PMCID: PMC571552210.7150/ijbs.2163529209143 [Google Scholar] [CrossRef] [PubMed]
[15]. Mellemkjaer L, Friis S, Olsen JH, Scélo G, Hemminki K, Tracey E, Risk of second cancer among women with breast cancerInt J Cancer 2006 118(9):2285-92.10.1002/ijc.2165116342146 [Google Scholar] [CrossRef] [PubMed]
[16]. Sakellakis M, Peroukides S, Iconomou G, Boumpoucheropoulos S, Kalofonos H, Multiple primary malignancies: A report of two casesChin J Cancer Res 2014 26(2):215-18.10.3978/j.issn.1000-9604.2014.02.15 [Google Scholar] [CrossRef]
[17]. Hemminki K, Aaltonen L, Li X, Subsequent primary malignancies after endometrial carcinoma and ovarian carcinomaCancer 2003 97(10):2432-39.10.1002/cncr.1137212733142 [Google Scholar] [CrossRef] [PubMed]
[18]. Fletcher O, Houlston RS, Architecture of inherited susceptibility to common cancerNat Rev Cancer 2010 10(5):353-61.10.1038/nrc284020414203 [Google Scholar] [CrossRef] [PubMed]
[19]. Takalkar U, Asegaonkar BN, Kodlikeri P, Asegaonkar S, Sharma B, Advani SH, An elderly woman with triple primary metachronous malignancy: A case report and review of literatureInt J Surg Case Rep 2013 4(7):593-96.10.1016/j.ijscr.2013.03.03223702365 [Google Scholar] [CrossRef] [PubMed]
[20]. Banimostafavi ES, Tayebi S, Tayebi M, Montazer F, Case Report: Synchronous primary malignancy including the breast and endometriumF1000Res 2017 6:1502PMCID: PMC575474710.12688/f1000research.11971.329333235 [Google Scholar] [CrossRef] [PubMed]
[21]. Cetinkaya K, Yüce E, Triple synchronous primary ovarian, endometrial and breast cancer: A case reportThe Journal of Gynecology- Obstetrics and Neonatology 2019 16:180-82. [Google Scholar]
[22]. Gaube A, Michire A, Văcăroiu IA, Andra-Elena BS, Georgescu D, Georgescu MT, Challenges in treatment and the importance of radiotherapy in a synchronous endometrial and breast cancerInternal Medicine 2022 19(4):89-97.10.2478/inmed-2022-0232 [Google Scholar] [CrossRef]