Unveiling the Cytological Enigma of Malignant Spindle Cell Neoplasm of Peripheral Nerve Sheath: A Clinicopathological Image
Shreya Giri Goswami1, Poornima Pandey2, Jayashree Bhawani3
1 Junior Resident, Department of Pathology, Datta Meghe Institute of Higher Education and Research, Wardha, Maharashtra, India.
2 Senior Resident, Department of Pathology, Datta Meghe Institute of Higher Education and Research, Wardha, Maharashtra, India.
3 Junior Resident, Department of Pathology, Datta Meghe Institute of Higher Education and Research, Wardha, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shreya Giri Goswami, Junior Resident, Department of Pathology, Datta Meghe Institute of Higher Education and Research, Wardha, Maharashtra, India.
E-mail: shreya18g@gmail.com
Cytomorphology, Fine needle aspiration cytology, Forearm swelling, Malignant mesenchymal tumour, Peripheral nerve sheath tumour, Soft-tissue tumour
Peripheral Nerve Sheath (PNS) tumours are rare neoplasms arising from deeper soft tissues, peripheral nerves, perineural cells, or Schwann cells with ectomesenchymal origins [1]. The incidence rate is between 4-13%, with the upper extremity being the most commonly involved site (45%) [2]. The role of Fine Needle Aspiration Cytology (FNAC), based on the cytomorphology of these neoplasms of the PNS, is challenging and often confusing, underscoring the complex diagnostic nature of the lesions. Therefore, FNAC details were highlighted by presenting a clinicopathological image and providing insight into the intricate cytomorphology involved.
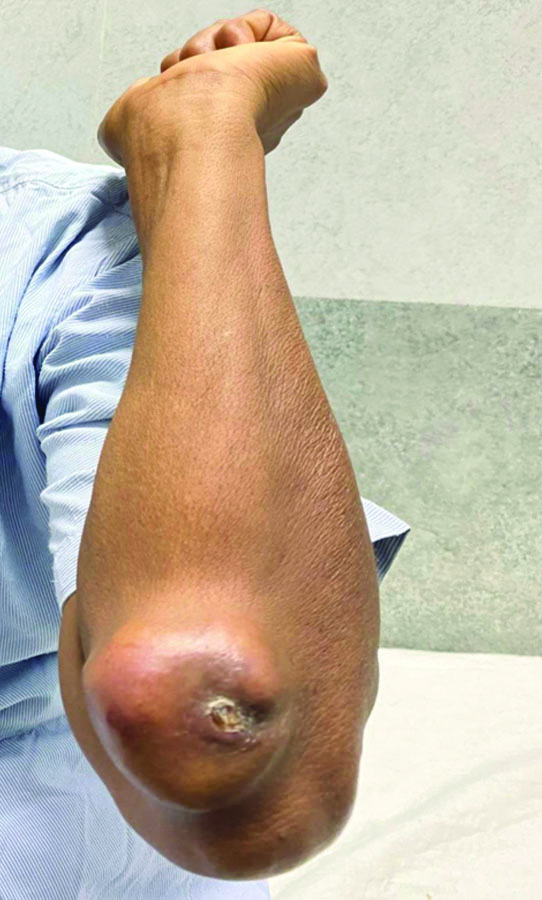
A 55-year-old woman presented to the surgery outpatient department with complaints of swelling near the elbow joint of her left forearm, accompanied by dull, aching pain for one year [Table/Fig-1]. The swelling was initially small, but over 10 months, it gradually increased to its current size. There was no history of locoregional trauma or falls. Upon locoregional examination, a 5×4 cm nodular swelling was observed, which was firm, fixed and tender, with surface ulceration of the skin present on the extensor aspect of the forearm, distal to the left elbow joint. There was no local temperature rise or involvement of the ulnohumeral joint. The general examination did not reveal any swelling at other sites. She did not provide any history of prior surgeries. The levels of electrolytes were within normal limits, and the serum urea and creatinine levels indicated normal kidney function.
Clinical picture showing a nodular swelling on the left forearm.
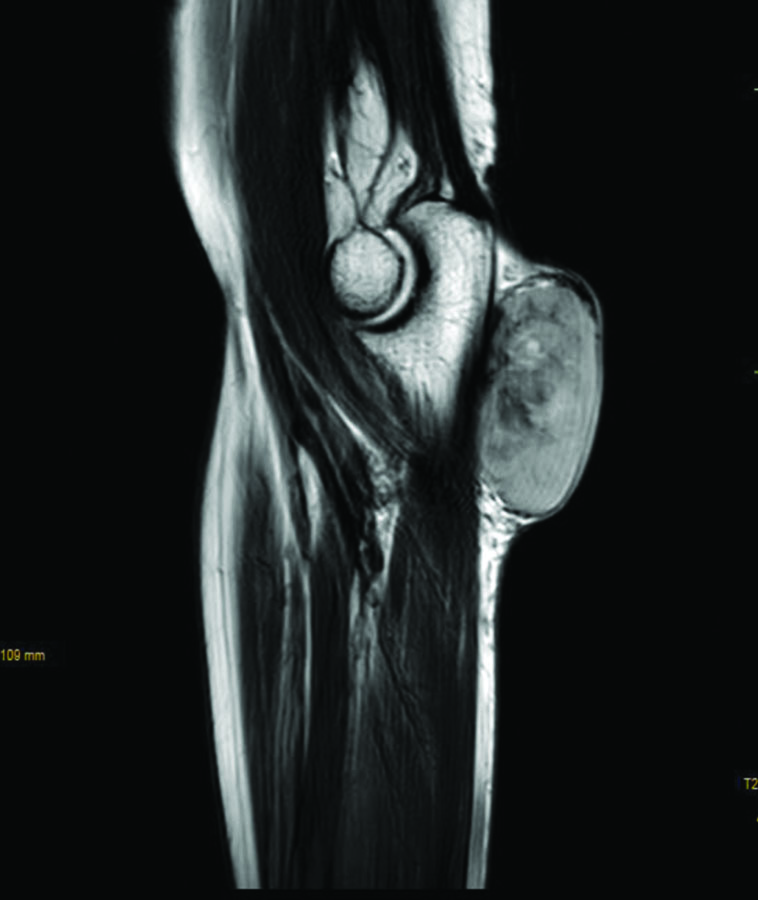
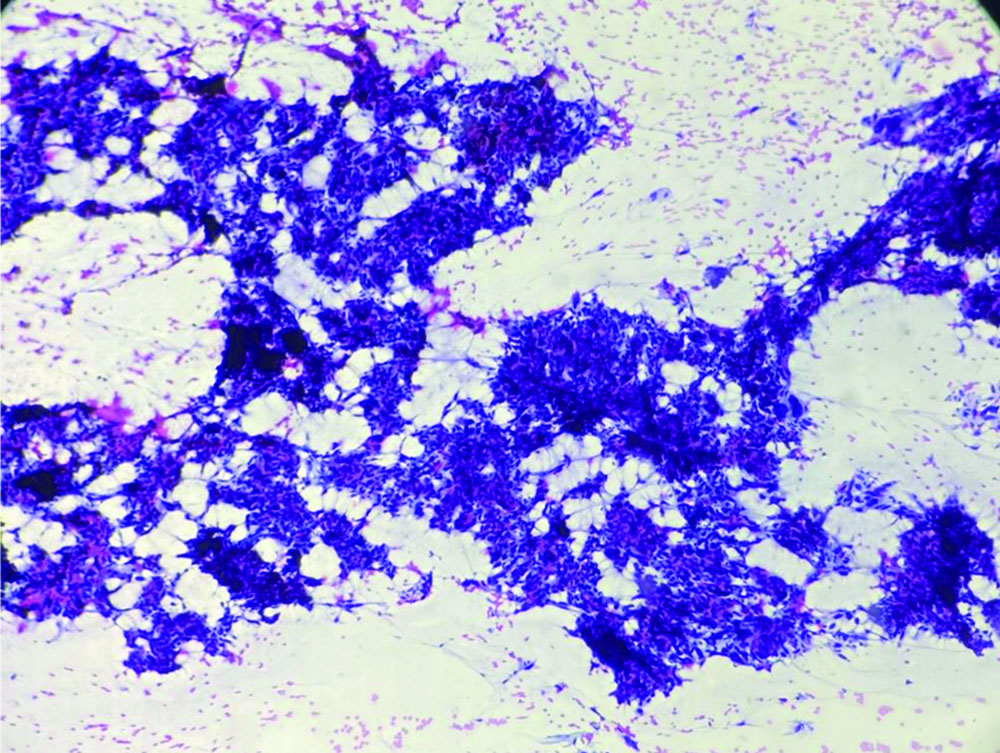
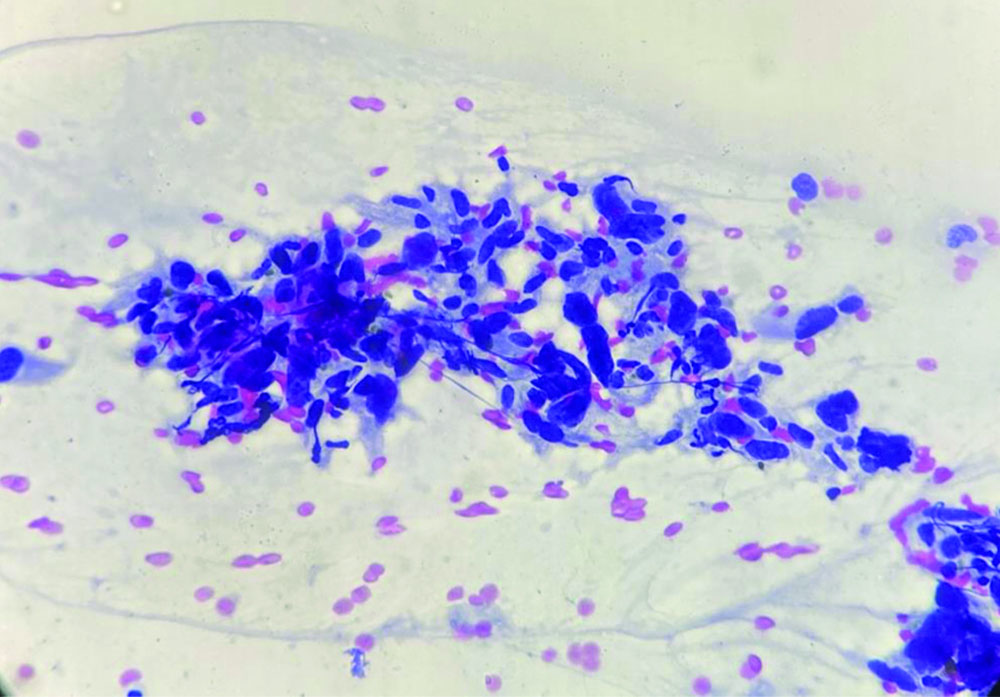
The patient underwent Ultrasonography (USG) of the left forearm, which showed a well-defined, heterogeneously hyperechoic lesion in the subcutaneous plane. There was no evidence of bony involvement and a radiodiagnosis of a soft-tissue tumour was given. Resonance Imaging (MRI) revealed a well-defined ovoid mass in the subcutaneous plane. The lesion was heterogeneously hyperintense with areas of central necrosis [Table/Fig-2]. The differential diagnosis included fibrosarcoma, dermatofibrosarcoma protuberans, poorly differentiated carcinoma, malignant fibrous histiocytoma nerve sheath tumour, along with the need for FNAC correlation. FNAC was carried out under USG guidance. The smears were of high cellularity, revealing multiple fragments of spindle nuclear cells [Table/Fig-3]. A few fragments showed peripheral nuclear palisading and attempted whorling. In some areas, the cell borders exhibited feathery margins [Table/Fig-4]. These elongated spindle nuclei showed moderate pleomorphism and were hyperchromatic. A few cells demonstrated bipolar blunting of nuclei and prominent nucleomegaly with uneven chromatin [Table/Fig-5]. The cytoplasm of these cells appeared to merge with intracellular hyalines. There were also a few spindle bare nuclei with atypia and infrequent mitosis in the background. The diagnosis of malignant spindle cell neoplasm of peripheral nerve sheath tumour (MPNST) was offered.
MRI showing well-defined hyperintense subcutaneous lesion.
FNA smear shows fragments and clusters of pleomorphic spindle nuclear cells (Papanicolaou stain, 10x).
FNA smear shows indistinct and feathery cytoplasmic borders (Papanicolaou stain, 40x).
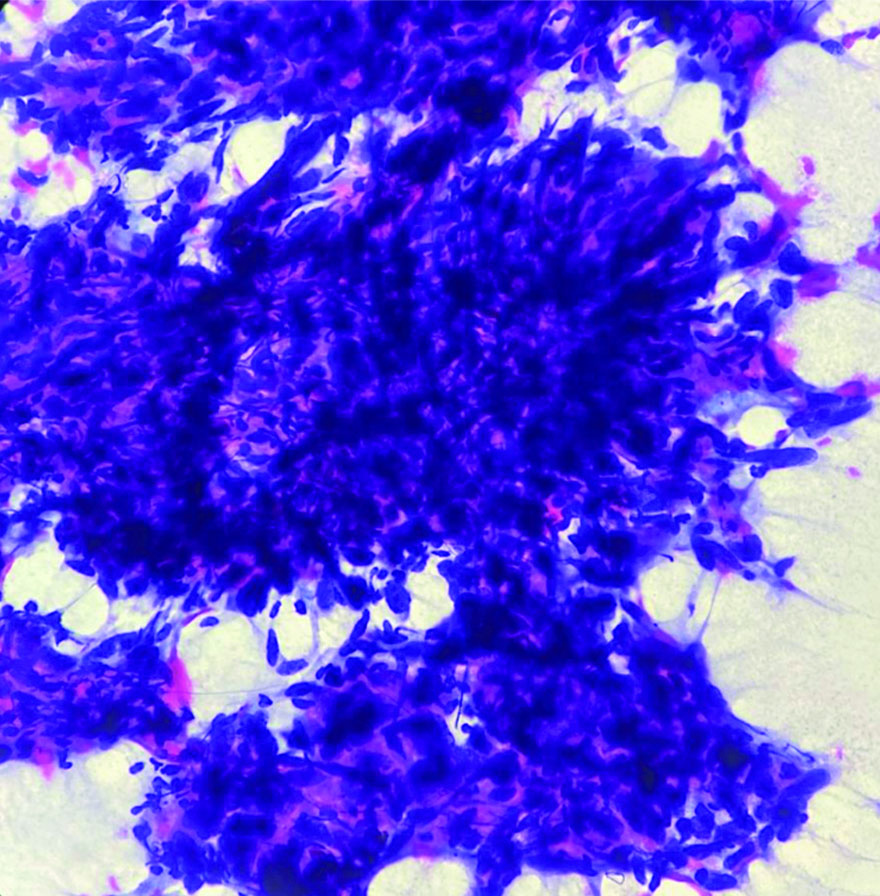
FNA smear shows polygonal-shaped highly pleomorphic nuclei with standout nucleomegaly (Papanicolaou stain, 40x).
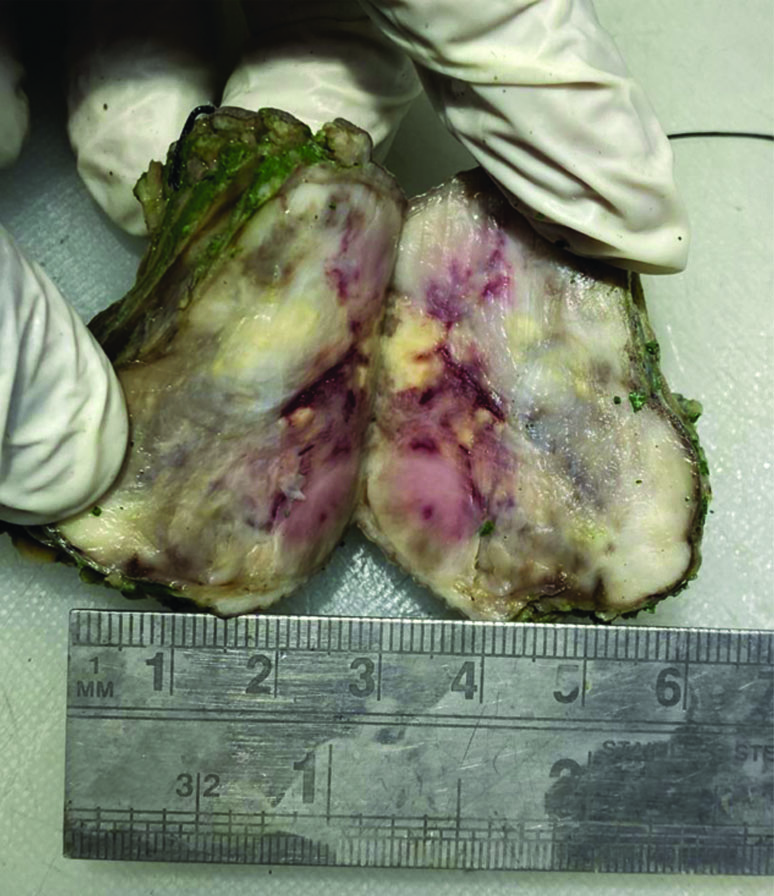
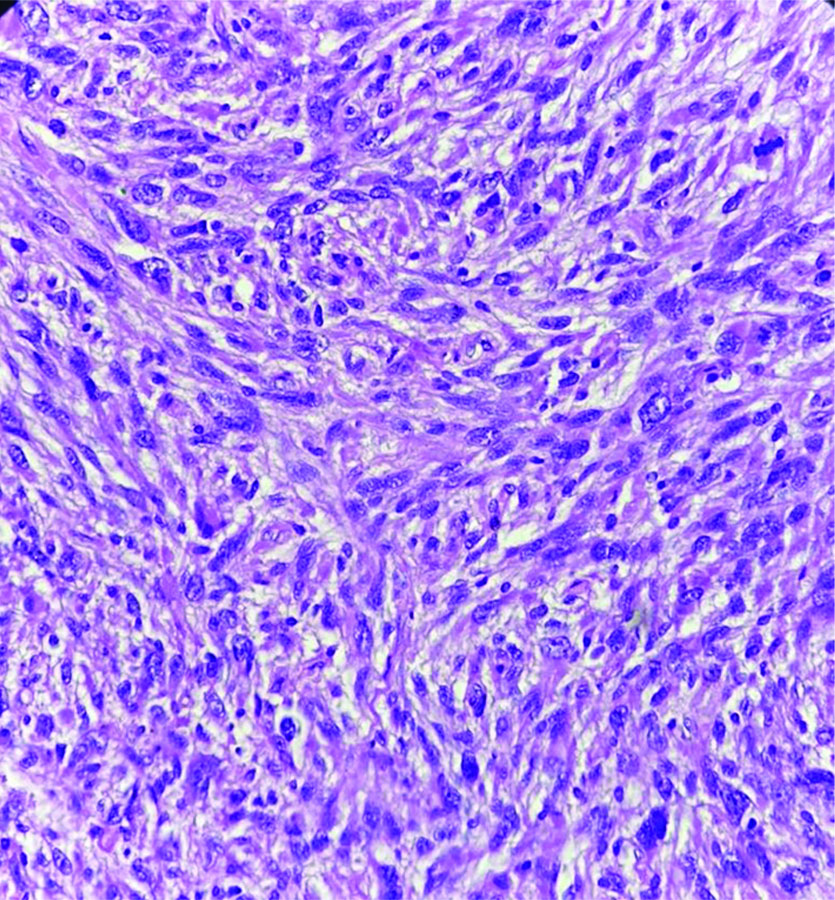
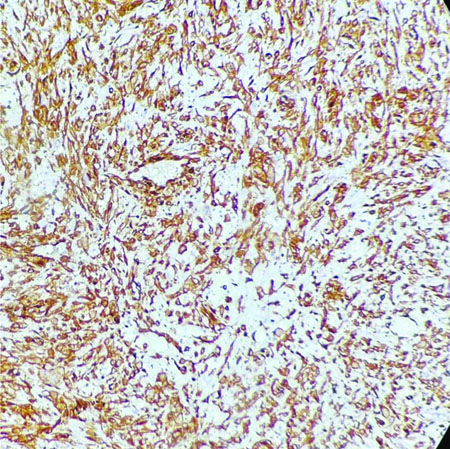
Following the cytodiagnosis, the patient underwent surgical resection of the tumour [Table/Fig-6]. The histopathology revealed pleomorphic spindle cells arranged in a fascicular arrangement. These cells exhibited scant wavy cytoplasm with marked anisonucleosis and a high N/C ratio. The hyperchromatic nuclei contained loose open chromatin, with nucleoli present in some. Mitotic figures were also observed. The histopathological features were suggestive of a malignant mesenchymal tumour [Table/Fig-7]. Immunohistochemical analysis confirmed the diagnosis of MPNST, showing diffuse positive immunoexpression for S-100 [Table/Fig-8] and SOX-10. The patient was followed-up by the Institute’s tumour board committee and was initiated on postoperative radiotherapy and chemotherapeutic drugs such as doxorubicin and ifosfamide as part of the adjunctive treatment plan.
The gross cut section of the tumour mass shows solid heterogenous whitish brown areas.
Section shows hypercellular neoplasm composed of pleomorphic spindle cells with a high N:C ratio. Occasional mitotic figures are seen (H&E, 40x).
Immunohistochemical evaluation of S-100 shows diffuse cytoplasmic positivity (IHC, 10x).
Discussion
Spindle cell tumours of the Peripheral Nervous System (PNS) are malignant, aggressive soft-tissue tumours that exhibit more than one line of cellular differentiation [3]. These tumours have ectomesenchymal origin and constitute approximately 5-10% of all soft-tissue tumours [1]. They commonly occur in individuals aged 20 to 63 years, with a predominance in females. The presented case involved a 55-year-old female, which aligns with findings from other studies. Dhingra KK et al., reported MPNST of the breast in a 38-year-old female [1]. Jimenez-Heffernan JA et al., conducted a cytomorphological study on 10 patients diagnosed with MPNST, noting a predominance of female patients, with ages ranging from 20 to 86 years [4]. Gupta K et al., retrospectively analysed eight histopathologically confirmed cases of MPNST, observing a male-to-female ratio of 1:3, with ages ranging from 22 to 60 years [5]. Parikh S et al., reported a case of a thigh mass diagnosed as MPNST in a 35-year-old female [6]. However, a large study conducted by Ducatman BS et al., on 120 cases of MPNSTs revealed that the mean age of occurrence was 34 years [7]. Trunks and extremities are the most common sites of involvement [2,3,6-9]. However, occurrences at rare sites such as the breast, parotid gland, and tongue have also been reported in the past [1,10,11].
MPNSTs, as the malignant counterparts of Schwannomas and Neurofibromas, sometimes closely emulate their clinical trajectories [3,7]. Consequently, preoperative diagnosis based on Fine Needle Aspiration (FNA) cytomorphological details becomes critical. However, the co-existence of various peripheral nerve lesions can result in overlapping cytological features, making it a diagnostic dilemma. Moreover, there is a limited number of Indian studies on the topic due to relatively rare exposure to such lesions [1,3,5,6].
There are few studies in the literature where cytodiagnosis of MPNST has been made [3-6,10-12]. The observations made by the aforementioned authors detailed the findings as follows: i) highly cellular smears; ii) large sheets and groups of elongated, plump spindle cells; iii) cell clusters that also showed nuclear palisading in some cases; iv) ill-defined and merging cytoplasmic borders; v) wavy and slender nuclei with moderate to severe pleomorphism, containing uniform chromatin with prominent nucleoli; vi) mitotic figures observed in a few cases; and vii) a myxoid background consisting of fine, fibrillary stroma.
Wakely PE et al., detailed cytomorphological features in 55 cases diagnosed with MPNST [13]. They described cellular smears containing syncytial clusters and singly dissociated populations of uneven-sized cells. These cells were elongated, with some oval cells having smooth borders but inconsistently tapered. The cells contained granular, dispersed chromatin and there was a fibrillary metachromatic stroma in the background.
The cytomorphological features observed in the present case for diagnosing the malignant spindle cell type of PNST were similar to those in the studies mentioned above.
The complexity in cytodiagnosis arises due to overlapping cytomorphological features among soft-tissue neoplasms. The diagnostic challenge is posed due to the varied cytomorphological mimicry, differentiation and heterogeneity. However, FNAC plays a key role in the preoperative diagnosis of soft-tissue tumours. A timely and accurate preoperative cytodiagnosis can facilitate early decision-making and prompt initiation of treatment.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 24, 2024
Manual Googling: Oct 19, 2024
iThenticate Software: Oct 22, 2024 (5%)
[1]. Dhingra KK, Mandal S, Roy S, Khurana N, Malignant peripheral nerve sheath tumor of the breast: Case reportWorld J Surg Onc 2007 5(1):14210.1186/1477-7819-5-14218154670PMC2246134 [Google Scholar] [CrossRef] [PubMed]
[2]. Arealis G, Kazamias K, Malik Tabassum K, Ashwood N, Malignant peripheral nerve sheath tumor of the forearm presenting as foreign bodyCureus 2021 13(5):e1522910.7759/cureus.15229 [Google Scholar] [CrossRef]
[3]. Gupta RK, Saran RK, Ghuliani D, Garg L, Das A, Metastatic epithelioid malignant peripheral nerve sheath tumor in a known case of neurofibromatosis-1, cytomorphological appearance, and critical analysis of immunohistochemistryIndian J Med Paediatr Oncol 2017 38(03):387-90.10.4103/ijmpo.ijmpo_113_1729200699PMC5686992 [Google Scholar] [CrossRef] [PubMed]
[4]. Jiménez-Heffernan JA, López-Ferrer P, Vicandi B, Hardisson D, Gamallo C, Viguer JM, Cytologic features of malignant peripheral nerve sheath tumorActa Cytol 1999 43(2):175-83.10.1159/00033097310097706 [Google Scholar] [CrossRef] [PubMed]
[5]. Gupta K, Dey P, Vashisht R, Fine-needle aspiration cytology of malignant peripheral nerve sheath tumorsDiagn Cytopathol 2004 31(1):01-04.10.1002/dc.2007915236255 [Google Scholar] [CrossRef] [PubMed]
[6]. Parikh S, Patel K, Shah B, Chaudhari V, Malignant peripheral nerve sheath tumor of thigh region-A case reportIAIM 2018 5(10):142-46. [Google Scholar]
[7]. Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM, Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 casesCancer 1986 57(10):2006-21.10.1002/1097-0142(19860515)57:10<2006::AID-CNCR2820571022>3.0.CO;2-63082508 [Google Scholar] [CrossRef] [PubMed]
[8]. Nascimento R, Valença-Filipe R, Horta R, Silva Á, Arm malignant peripheral nerve sheath tumour: A rare clinical presentationBMJ Case Rep 2019 12(8):e22985210.1136/bcr-2019-22985231420428PMC6700586 [Google Scholar] [CrossRef] [PubMed]
[9]. Abdel Al S, Abou Chaar MK, Asha W, Al-Najjar H, Al-Hussaini M, Fungating malignant peripheral nerve sheath tumor arising from a slow-growing mass in the forearm: A case report and review of the literatureJ Med Case Reports 2020 14(1):9110.1186/s13256-020-02427-432631436PMC7339469 [Google Scholar] [CrossRef] [PubMed]
[10]. Nepka C, Karadana M, Karasavvidou F, Barbanis S, Kalodimos G, Koukoulis G, Fine needle aspiration cytology of a primary malignant peripheral nerve sheath tumor arising in the parotid glandActa Cytol 2009 53(4):423-26.10.1159/00032534419697728 [Google Scholar] [CrossRef] [PubMed]
[11]. Ashraf MJ, Azarpira N, Hashemi SB, Fine needle aspiration cytology of malignant peripheral nerve sheath tumor of the tongueActa Cytol 2010 54(1):117-19.10.1159/00032498520307006 [Google Scholar] [CrossRef] [PubMed]
[12]. Jiwani S, Gokden M, Lindberg M, Ali S, Jeffus S, Fine-needle aspiration cytology of epithelioid malignant peripheral nerve sheath tumor: A case report and review of the literatureDiagn Cytopathol 2016 44(3):226-31.10.1002/dc.2340126646298 [Google Scholar] [CrossRef] [PubMed]
[13]. Wakely PE, Ali SZ, Bishop JA, The cytopathology of malignant peripheral nerve sheath tumor: A report of 55 fine-needle aspiration casesCancer Cytopathol 2012 120(5):334-41.10.1002/cncy.2119522434579 [Google Scholar] [CrossRef] [PubMed]