Case Report
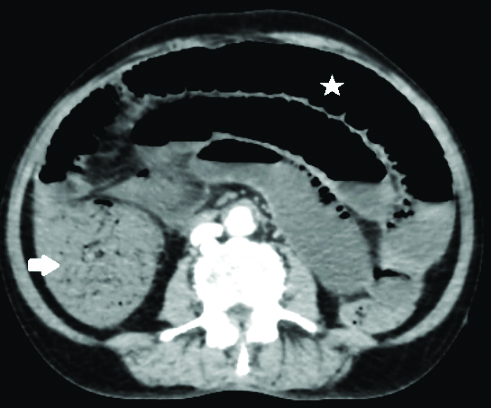
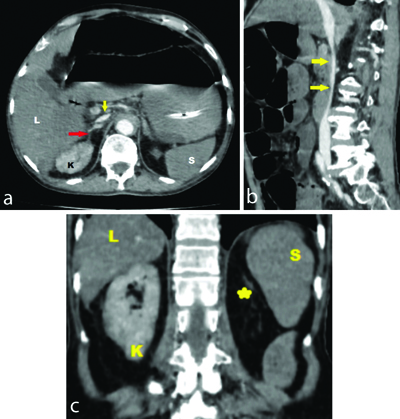
A 78-year-old male patient presented to the Emergency Medicine Department with abdominal pain and distension for two days, along with two episodes of vomiting over the past day. His blood pressure upon arrival was low, measuring 90/60 mmHg and his heart rate was 90 beats per minute. An abdominal examination revealed a distended abdomen with tenderness in all four quadrants. On auscultation, bowel sounds were sluggish. Due to the abdominal complaints and to avoid any delay in diagnosis, patient was sent directly for a CECT of the abdomen without any oral contrast. The CECT abdomen revealed diffusely dilated small bowel loops and an ascending colon [Table/Fig-1]; however, no wall thickening or hyperenhancement of the bowel wall was present. Signs of hypovolemia were observed [Table/Fig-2a-c] in the form of a flattened IVC, as well as, hypoenhancing liver, spleen and pancreas. Hyperenhancement was noted only in the right adrenal gland. Additionally, we found that the patient had only one kidney on the right-side, with an absent left kidney. The right kidney showed heterogeneous hyperenhancement.
Axial image CECT abdomen reveals dilated small bowel loops (asterisk) and ascending colon (white arrow), however no abnormal wall thickness and enhancement was seen.
a) Axial image; and b) sagittal image CECT abdomen- shows flattened Inferior Vena Cava (IVC) (yellow arrow) and hyperenhancing right adrenal gland (red arrow). a) (axial image), c) (coronal image) shows reduced enhancement of liver (L) and spleen (S) with increased enhancement of right kidney (K). Note empty left renal fossa (yellow star).
Based on the clinical and radiological findings, a diagnosis of CT Hypoperfusion Complex (CHC) was made. Based on clinical findings alone, management with nasal oxygen therapy and resuscitation with intravenous fluids was initiated. However, the patient succumbed and passed away after a few hours. This can be attributed to the advanced age of the patient, a delay in seeking medical treatment and a poor general condition at the time of presentation to the hospital.
Discussion
Shock is a life-threatening manifestation of circulatory failure, characterised by cellular and tissue hypoxia resulting from reduced oxygen (O2) supply, increased O2 consumption, or inadequate utilisation of O2. The most common manifestation of hypotension is defined as a systolic blood pressure of less than 90 mmHg [1]. Depending on the aetiology, shock has been categorised into four types: Hypovolemic Shock (HS), Distributive Shock (DS), Cardiogenic Shock (CS) and Obstructive Shock (OS) [2,3]. Regardless of the aetiology, the outcome of shock is hypoperfusion and multiorgan failure due to a disproportionate oxygen demand and supply [3,4]. Hypoperfusion of vital organs produces distinct signs on CECT imaging, now referred to as the CHC or Hypovolemic Shock Complex (HSC) [5,6]. Initially reported in cases of HS secondary to traumatic haemorrhage, these findings have later been documented in cases of shock due to sepsis, myocardial infarction and diabetic ketoacidosis [5-7]. Recognising these imaging signs by radiologists assists physicians in making early and appropriate interventions and treatments for patients. The CHC signs are observed in CECT scan images, particularly in the vessels and organs of the abdomen (and sometimes in the thorax) in patients with shock [5]. The spectrum of CT findings can be categorised into vascular and visceral (both solid and hollow viscera) signs. The presence of two or more of these signs is diagnostic of CHC [5,8]. The vascular signs are thought to result from the hypovolemic state, while hypoperfusion produces visceral signs [7,9].
Vascular signs: The vascular signs include the flattening of the IVC, which is defined as an Anteroposterior Diameter (APD) of the IVC measuring less than 9 mm in three consecutive segments (the intrahepatic portion of the IVC, 2 cm above and below the renal veins) [5,7-10]. Another sign, known as the ‘halo sign,’ refers to low-attenuating fluid (<20 HU) surrounding a collapsed IVC, which can also be observed in a few cases [7,8,10]. Although variations in intra-abdominal pressure and the respiratory cycle can affect the IVC diameter, many studies have documented that flattening of the IVC has a specificity of 90% and a sensitivity of 84% in diagnosing hypoperfusion shock in patients with spontaneous breathing [6,7]. Flattening of the IVC was clearly demonstrated in the present case. Similarly, a reduced APD (<13 mm) of the aorta, observed 2 cm above and below the renal arteries, although not specific, is another vascular sign that can be demonstrated in a few cases [5,7,8,10].
Hollow visceral signs: The most frequently and widely reported CT signs of hypovolemia involving hollow visceral organs are the abnormalities seen in the small bowel [9,11]. The common findings, termed ‘shock bowel,’ comprise abnormalities such as abnormal small bowel wall thickening (>3 mm) and dilatation (>2.5 cm) with abnormally increased postcontrast wall enhancement (HU>psoas muscle) [3,5-11]. The large bowel is found to be infrequently involved [7,8,10]. The mechanism of shock bowel is thought to be secondary to hypovolemia, which triggers sympathetic stimulation, resulting in splanchnic vasoconstriction and decreased bowel perfusion [6,7,11]. Severe hypotension, which causes insufficient oxygen supply to end organs, alters bowel permeability, resulting in fluid and contrast media leakage into the interstitial space, leading to prominent mucosal enhancement and submucosal wall oedema. Bowel dilatation may occur due to ileus and poor fluid resorption [6,7,11]. Shock bowel, initially identified in post-traumatic patients with hypovolemia, was later documented by Di Serafino M et al., in their study, which found shock bowel signs in patients experiencing shock due to other reasons [7].
Solid Visceral Signs
Liver: Abnormal or heterogeneous postcontrast parenchymal enhancement is observed in the liver, termed ‘shock liver’ [6-10]. The reduced enhancement is attributed to the portal vein having less sympathetic activity than other intra-abdominal vessels. Intrahepatic increased vascular enhancement and intrahepatic perivascular oedema have also been documented [8,10]. The gallbladder, although with low specificity, may also show manifestations of shock through abnormal hyperenhancement of the wall without any wall thickening [3,6-8]. In the present case, the liver exhibited hypoenhancement.
Spleen: Typically, splenic postcontrast enhancement is greater than that of the liver. However, in shock situations secondary to hypoperfusion, splenic postcontrast enhancement is less than that of the liver. When compared with the liver, if the spleen shows decreased enhancement of >30 HU in children and >20 HU in adults, it is considered hypoenhancement of the spleen [3,6-8]. The autoregulatory mechanism is lacking in the splenic arterial system, rendering it sensitive to overstimulation of the sympathetic system and vasoconstriction, which results in decreased splenic blood flow and hypoenhancement [3,7]. A decrease in splenic volume of 30% or more compared to normal values is defined as a contracted spleen [3,8,9]. This phenomenon is attributed to a process known as ‘auto-transfusion,’ where, to compensate for the state of hypovolemia, stimulation of sympathetic efferent nerves (which innervate the smooth muscles of vessel walls and the splenic capsule) occurs. This stimulation causes contraction, expelling red blood cells and platelets into the general circulation and increasing sympathetic-driven venous return to the heart [3,12]. In the current case, the spleen demonstrated hypoenhancement.
Kidneys: Increased and prolonged cortical enhancement is noted in the kidneys in cases of hypovolemia [3,5-8]. The renal cortex shows greater enhancement compared to the aorta in the portal venous phase [9] and is referred to as ‘white kidneys’ [6]. The hyperenhancement is thought to be secondary to the activation of the renin-angiotensin axis following reduced blood flow to the kidneys, causing a dramatic increase in angiotensin II levels and constriction of renal efferent arterioles [3,6-8]. However, in cases of severe hypovolemia, absence or hypoenhancement (defined as enhancement <10 HU) may also occur and is termed the ‘black kidney sign’ [6,8,11]. In the present case, the patient had a single kidney, which showed heterogeneous hyperenhancement in the venous phase but demonstrated lesser enhancement compared to the aorta.
Adrenals: One of the earliest CT signs of shock, especially in cases of Circulatory Shock (CS), can be observed as symmetrical intense enhancement (equal to or greater than that of the IVC of the bilateral adrenals in the portal venous phase [3,5-9]. Hypotension results in stimulation of the hypothalamus-pituitary axis and subsequent overactivity of the sympathetic system, leading to increased levels of nor adrenaline and angiotensin II, which redirect blood flow to the adrenals [6,7]. In cases of septic shock, a common and specific finding observed on dual-phase CECT has been recently documented in the literature. This finding is called the ‘hollow adrenal gland sign,’ which is defined as the central adrenal zone showing less intense enhancement compared to the peripheral zone in the arterial phase, followed by homogeneous enhancement in the venous phase [6,13]. Adrenal hyperenhancement is a frequent sign of hypovolemia and is seen in up to 59% of cases with severe hypovolemia [8]. In the present case, the adrenals exhibited symmetrical hyperenhancement comparable to that of the IVC.
Pancreas: Variable pancreatic enhancement (both hyperenhancement and hypoenhancement) with peripancreatic oedema is observed in patients with Chronic Hypoperfusion Conditions (CHC) and is termed ‘shock pancreas’ [3,6-8].
Other Manifestations
Thyroid: Only a few cases in the literature describe signs of CHC in the thyroid gland. The thyroid gland shows an increase in size and heterogeneous enhancement, a condition referred to as ‘shock thyroid’ [6,7,11].
Thoracic findings: In a limited number of patients, haemodynamic instability and injury have been documented in the literature. These findings include a reduced thoracic aortic caliber with increased enhancement, a reduced caval caliber and areas of pulmonary contusion and/or collapse showing increased parenchymal enhancement [8].
Conclusion(s)
It is now possible to diagnose changes in shock using CECT imaging. Recognising these signs can help radiologists and clinicians take timely action for appropriate management.
[1]. Haseer Koya H, Paul M, Shock. [Updated 2023 Jul 24]In: StatPearls [Internet] 2024 Jan Treasure Island (FL)StatPearls PublishingAvailable from: https://www.ncbi.nlm.nih.gov/books/NBK531492/ [Google Scholar]
[2]. Taghavi S, Nassar Ak, Askari R, Hypovolemic Shock. [Updated 2023 Jun 5]In: StatPearls [Internet] 2024 Jan Treasure Island (FL)StatPearls PublishingAvailable from: https://www.ncbi.nlm.nih.gov/books/NBK513297/ [Google Scholar]
[3]. Počepavičiūtė K, Pavilionė R, CT hypoperfusion complex: How to recognize shock in the absence of profound hypotensionRadiol Update 2021 5:13-20. [Google Scholar]
[4]. Standl T, Annecke T, Cascorbi I, Heller AR, Sabashnikov A, Teske W, The nomenclature, definition and distinction of types of shockDtschArztebl Int 2018 115(45):757-68.10.3238/arztebl.2018.075730573009PMC6323133 [Google Scholar] [CrossRef] [PubMed]
[5]. Yüce İ, CT hypoperfusion complex: Emergency ct results during one yearEurasian J Emerg Med 2016 15:136-38.10.5152/eajem.2016.32932 [Google Scholar] [CrossRef]
[6]. Valente T, Bocchini G, Massimo C, Rea G, Lieto R, Guarino S, Multidetector CT imaging biomarkers as predictors of prognosis in shock: Updates and future directionsDiagnostics (Basel) 2023 13(13):230410.3390/diagnostics1313230437443697PMC10341185 [Google Scholar] [CrossRef] [PubMed]
[7]. Di Serafino M, Viscardi D, Iacobellis F, Giugliano L, Barbuto L, Oliva G, Computed tomography imaging of septic shock. Beyond the cause: The “CT hypoperfusion complex”. A pictorial essayInsights Imaging 2021 12(1):7010.1186/s13244-021-01006-534089401PMC8178660 [Google Scholar] [CrossRef] [PubMed]
[8]. Wang J, Liang T, Louis L, Nicolaou S, McLaughlin PD, Hypovolemic shock complex in the trauma setting: A pictorial reviewCan Assoc Radiol J 2013 64(2):156-63.10.1016/j.carj.2013.03.00223608512 [Google Scholar] [CrossRef] [PubMed]
[9]. Elst J, Ghijselings IE, Zuidema WP, Berger FH, Signs of post-traumatic hypovolemia on abdominal CT and their clinical importance: A systematic reviewEur J Radiol 2020 124:108800Epub 2019 Dec 2410.1016/j.ejrad.2019.10880031935595 [Google Scholar] [CrossRef] [PubMed]
[10]. Tarrant AM, Ryan MF, Hamilton PA, Benjaminov O, A pictorial review of hypovolaemic shock in adultsBr J Radiol 2008 81(963):252-57.Epub 2008 Jan 710.1259/bjr/4096205418180262 [Google Scholar] [CrossRef] [PubMed]
[11]. Ames JT, Federle MP, CT hypotension complex (shock bowel) is not always due to traumatic hypovolemic shockAJR Am J Roentgenol 2009 192(5):W230-W235.10.2214/ajr.08.147419380528 [Google Scholar] [CrossRef] [PubMed]
[12]. Saxena M, Shour T, Shah M, Wolff CB, Julu POO, Kapil V, Attenuation of splanchnic autotransfusion following non-invasive ultrasound renal denervation: A novel marker of procedural successJ Am Heart Assoc 2018 7(12):e009151Published 2018 Jun 1210.1161/JAHA.118.00915129895590PMC6220552 [Google Scholar] [CrossRef] [PubMed]
[13]. Costa A, Baptista M, The hollow adrenal gland sign: An ominous alertJ Belg Soc Radiol 2022 106(1):61Published 2022 Jun 2810.5334/jbsr.283035854823PMC9248986 [Google Scholar] [CrossRef] [PubMed]