Periapical lesions result from endodontic infections, leading to inflammation and bone resorption due to microbial and host defense interactions at the necrotic root canal and periodontal tissue interface [1]. Mechanical debridement and chemical irrigation typically achieve an 85% success rate in treating periapical lesions. However, 10-15% of cases fail, necessitating surgical intervention [2]. Surgical endodontics addresses lesions that are unresponsive to conventional therapy.
Periapical surgery aims to remove pathology and regenerate bone and periodontal tissue [3]. The healing outcome depends on wound nature of the wound, progenitor cells, signalling molecules and the microenvironment, resulting in either repair or regeneration [4]. Regeneration restores tissue architecture and function, while repair does not. Various materials can fill bone defects, including gelatin sponges, fibrin preparations and bone grafts [5].
Sticky bone, introduced by Sohn DS et al., is a growth factor-enriched bone graft matrix made using Autologous Fibrin Glue (AFG) [6]. It contains essential elements for bone formation and various growth factors, which accelerating tissue healing and minimising bone loss [7]. The fibrin network of sticky bone prevents scattering, supports bone stabilisation and promotes regeneration [7].
Guided Tissue Regeneration (GTR) techniques use barrier membranes to regenerate bone and periodontal tissue. Introduced by Nyman S et al., in 1982, GTR prevents epithelial cell migration into the wound space, allowing regenerative cells to proliferate [8,9]. Platelet-rich Fibrin (PRF), developed by Choukroun J et al., is a second-generation platelet concentrate that enhances wound healing, bone growth and graft stabilisation without biochemical handling [10,11].
Surgical intervention is essential when non surgical root canal therapy fails. GTR, sticky bone and PRF enhance bone regeneration and minimise complications in larger cysts, such as infection and clot breakdown [5,12]. Despite the promising results of using sticky bone, PRF and GTR membranes in endodontic surgeries, there is a lack of comprehensive studies evaluating their combined effects on the healing kinetics and regenerative outcomes in periapical tissues, highlighting the need for further research in this area. Therefore, the present study aimed to compare the radiological evaluation of healing kinetics and regenerative effects of PRF and GTR barrier membranes after periapical surgeries in apicoectomy cases. The comparison involves using sticky bone alone, sticky bone with GTR membrane and sticky bone with PRF as a barrier membrane and their impact on the healing of periapical tissues.
The null hypothesis for the present study was that there is no statistically significant difference in bony healing, specifically in the improvement of bone density and reduction of lesion size across the three groups: Group I (control with sticky bone), Group II (sticky bone with GTR membrane) and Group III (sticky bone with PRF membrane).
Materials and Methods
The present prospective, single-centre, randomised clinical trial was conducted in the Department of Conservative Dentistry and Endodontics, Dr. R. Ahmed Dental College and Hospital, Kolkata, West Bengal, India, from July 2022 to December 2023. The study received approval from the Institutional Ethics Committee (IEC).
Inclusion and Exclusion criteria: Inclusion criteria included patients in good general health, available for follow-up, willing to participate with informed consent and having small (5-<10 mm diameter [13]) to large periapical lesions (≥10-15 mm diameter [13,14]), confirmed by preoperative CBCT. Patients were excluded if they were psychologically compromised, suffered from severe systemic diseases, had known allergies or foreign body sensitivity, were unwilling to participate, or were elderly.
A pilot study was conducted over two months to estimate the effect size and validate the feasibility of the proposed methodology. The present study included 15 patients with periapical lesions, divided into three groups of five patients each. Group I received treatment with sticky bone only, Group II received treatment with sticky bone combined with a GTR membrane and Group III received treatment with sticky bone and PRF membrane alone. The primary objectives were to evaluate the preliminary effectiveness of these treatments in improving bone density and reducing periapical lesion size. Radiographic evaluations using CBCT were conducted at baseline and at the end of the two months. The pilot study revealed an effect size of 0.8 for differences between groups concerning lesion size reduction and bone density improvement. These preliminary findings helped in the sample size calculation for the main study and facilitated refinements in the study design and outcome measures.
Sample size calculation: Based on the pilot study results, the sample size for the main study was determined to be 24 subjects, with 8 subjects per group, using G*Power Software version 3.1.9.7. This calculation was based on an analysis of variance model with an effect size of 0.8, an alpha error of 0.05, a power of 80% and a two-tailed significance level (α) of 0.05. To account for a 20% dropout rate, the sample size was adjusted to 30 subjects, with 10 patients per group.
Study Procedure
Informed consent was obtained from all participating patients after the study procedures were explained in their native language, in according to the Helsinki Declaration. The radiographic evaluation began with Intraoral Periapical Radiographs (IOPAR) to assess initial periapical lesions. Subsequently, CBCT scans (Skyview 3D Panoramic Imager manufactured by MyRay Dental Imaging, Imola, Italy) were performed to analyse the three-dimensional dimensions of the periapical lesions and their bone density was measured in Hounsfield Units (HU). The digital imaging and communications in medicine format images were exported from the Skyview CBCT scanner and imported into the iRYS viewer software.
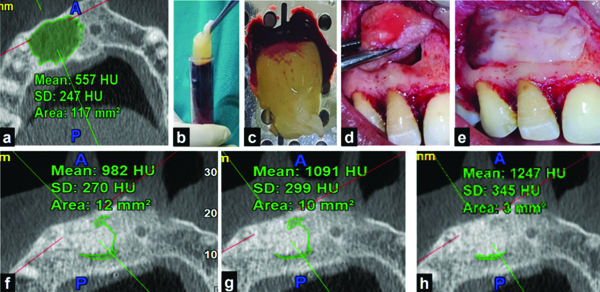
Following the radiographic assessment, root canals were obturated as per standard protocols, ensuring comprehensive baseline data collection and treatment within the study cohort. Endodontic microsurgery ensued with the administration of local anaesthesia (2% lignocaine with adrenaline 1:80,000) and the provision of an incision (one horizontal and two vertical releasing incisions), followed by the reflection of a full-thickness mucoperiosteal flap. Subsequently, an osteotomy window was prepared, the root apex was identified and 3 mm of the root end was resected. Enucleation of the lesion was performed, followed by the preparation of a retrograde cavity using an appropriate ultrasonic surgical tip and filling it with Mineral Trioxide Aggregate (MTA) (Angelus, Brazil). In Group I patients, sticky bone was placed in the bony cavity [Table/Fig-1].
Procedural steps in Group I: a) Preoperative Cone Beam Computed Tomographic (CBCT) scan; b) Resultant blood after centrifugation at 2700 rpm for 2 minutes; c) Hydroxyapatite (HA) granules; d) AFG retrieved from the topmost layer of the resultant blood mixed with HA granules; e) Sticky bone formed; f) Sticky bone placed in the bony socket; g) Postoperative CBCT scan at 3 months; h) Postoperative CBCT scan at 6 months; i) Postoperative CBCT scan at 12 months.
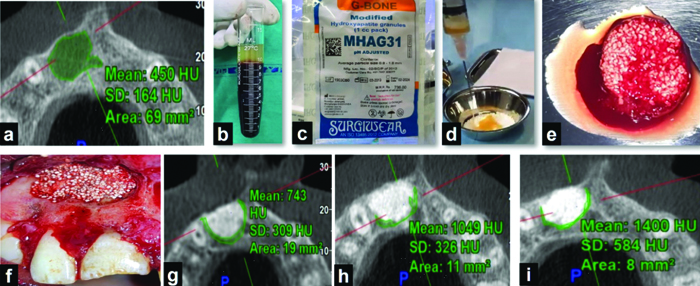
In Group II patients, after packing the bony cavity with sticky bone, the bony wound was covered with a resorbable GTR membrane (Healiguide, Advanced Biotechnologies, Inc., USA). For the preparation of sticky bone, under aseptic conditions, blood was drawn from the antecubital vein of the patients using disposable 10 mL syringes and transferred into glass test tubes without anticoagulant. The blood was then centrifuged at 2700 rpm for two minutes, resulting in two layers: the top layer containing AFG and the bottom layer consisting of Red Blood Corpuscles (RBC). AFG was collected from the base of the RBCs using a syringe and transferred into a sterile dappen dish. It was then mixed with hydroxyapatite crystals (Surgiwear) and left for 5-10 minutes to allow polymerisation to complete [Table/Fig-2].
Procedural Steps in Group II: a) Preoperative Cone Beam Computed Tomographic (CBCT) scan; b) Sticky bone placed in the bony socket; c) Guided tissue regeneration (GTR) membrane placed over sticky bone; d) Postoperative CBCT scan at 3 months; h) Postoperative CBCT scan at 6 months; i) Postoperative CBCT scan at 12 months.
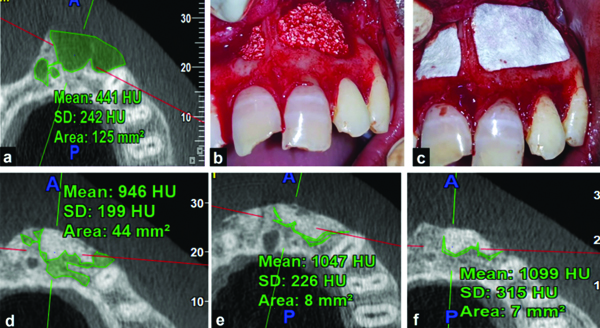
For Group III patients, after packing the bony cavity with sticky bone (prepared by the same aforementioned method), the bony wound was covered with a PRF membrane as a barrier membrane. PRF was prepared according to Choukroun’s protocol [10,11]. Blood was drawn from the patient’s antecubital vein and transferred into glass test tubes without anticoagulant. It underwent centrifugation at 400Xg or rcf (2114 rpm) for 10 minutes, yielding three layers: Platelet-Poor Plasma (PPP) on top, PRF in the middle (characterised by a clot with a high concentration of platelets) and RBC at the bottom. PRF was delicately separated from the RBC layer immediately after removing the PPP and then transferred into a PRF box for further processing. The PRF membrane was then prepared using the PRF box’s compression technique, ensuring gentle and uniform pressure [Table/Fig-3]. Following this, the flap was sutured with 3-0 black silk sutures and a specimen was collected in 10% formalin solution for histopathological examination. Patients were scheduled for subsequent follow-ups for evaluation of the outcome parameters.
Procedural steps in Group III: a) Preoperative Cone Beam Computed Tomographic (CBCT) scan; b) Resultant blood after centrifugation at 2114 rpm (400G) for 10 minutes, followed by retrieval of Platelet-rich Fibrin (PRF) from the intermediate layer of the resultant blood; c) PRF membrane prepared by compression technique; d) Sticky bone placed in the bony socket; e) PRF membrane placed over sticky bone; f) Postoperative CBCT scan at 3 months; g) Postoperative CBCT scan at 6 months; h) Postoperative CBCT scan at 12 months.
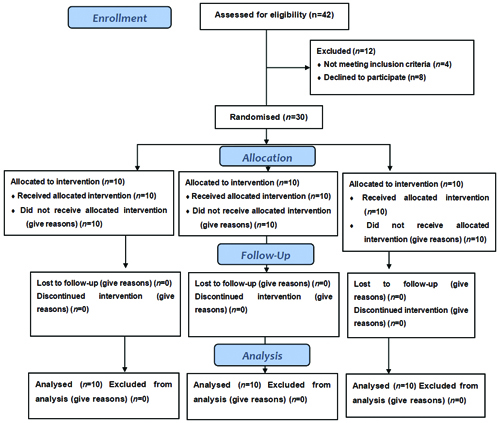
Participant flow: A Consolidated Standards of Reporting Trials (CONSORT) flow diagram [Table/Fig-4] is provided to illustrate the participant recruitment, allocation, follow-up and analysis stages of the study. This diagram visually represents the flow of participants through each stage of the study, from initial enrollment to final analysis and helps to clarify the process and any exclusions that occurred.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Parameters assessed: The outcome variables assessed in the present study included the reduction in lesion size (measured by the area of the lesion in mm2) and the increase in bone density, measured in Hounsfield Units (HU), at baseline (preoperatively) and 12 months postoperatively using iRYS viewer CBCT software. The software’s measurement tool was utilised to define the Region of Interest (ROI) manually for both area and bone density assessments. The software provided gray-level values to estimate bone density, enabling a precise evaluation of the healing process by comparing measurements at both time points.
Statistical Analysis
The data was tabulated and assessed by IBM SPSS Statistics for Windows, Version 27.0 (Armonk, NY: IBM Corp). The Chi-square test was used to evaluate the demographic variables. Statistical evaluation included a paired t-test and one-way Analysis of Variance (ANOVA) with post-hoc Tukey’s test, following confirmation of data normality. An alpha level of 5% was considered the level of statistical significance.
Results
The mean±Standard Deviation (SD) of participants whose teeth were included was 22.5±7.53 years for Group I, 25.60±7.198 years for Group II and 24.4±5.68 years for Group III, respectively. The mean age of the total study population was 24.16 years. Overall, there were 14 (46.7%) males and 16 (53.3%) females in the study. No significant differences were noted among the ages of the study participants (p=0.6) or the gender proportions (p=0.9) between the three groups, respectively indicating demographic equivalence between the groups [Table/Fig-5].
Demographic characteristics of the study subjects across all the study groups.
| Demographic characteristics | Group I(n=10) | Group II(n=10) | Group III(n=10) | Total(N=30) | p-value |
|---|
| Age (in years)a mean±Standard Deviation (SD) |
| 22.5±7.53 | 25.6±7.2 | 24.4±5.68 | 24.17±6.74 | 0.6 NS |
| Genderb n (%) |
| Male | 5 (50%) | 5 (50%) | 4 (40%) | 14 (46.7%) | 0.9 NS |
| Female | 5 (50%) | 5 (50%) | 6 (60%) | 16 (53.3%) |
n: Sample size per study group; N: Total sample size; a: values expressed as Mean (standard deviation) and analysed by the One-way ANOVA test; b: values expressed in frequencies (percentage) and analysed by the Chi-square test; NS: Not statistically significant (p>0.05)
The intragroup comparison regarding periapical lesion size (mm2) among the three groups revealed the following findings: In Group I, the mean±SD periapical lesion size was 91.59±64.39 mm2 preoperatively, showing a statistically significant reduction to 10.09±6.005 mm2 at the 12-month follow-up (p=0.002). In Group II, the preoperative mean±SD lesion size was 102.65±29.40 mm2, decreasing significantly to 7.23±4.06 mm2 at 12 months (p=0.001). Similarly, in Group III, the periapical lesion size decreased from a mean±SD of 111.235±40.49 mm2 preoperatively to 4.968±5.10 mm2 at 12 months (p<0.001) [Table/Fig-6].
Characteristics of the lesion size (in mm2) (preoperative, postoperative and percentage reduction) of all the study groups.
| Time points/Study groups | Group I(n=10) | Group II(n=10) | Group III(n=10) |
|---|
| Preoperative | 91.59±64.39 | 102.65±29.40 | 111.235±40.49 |
| Postoperative (12 mons) | 10.09±6.005 | 7.23±4.06 | 4.968±5.10 |
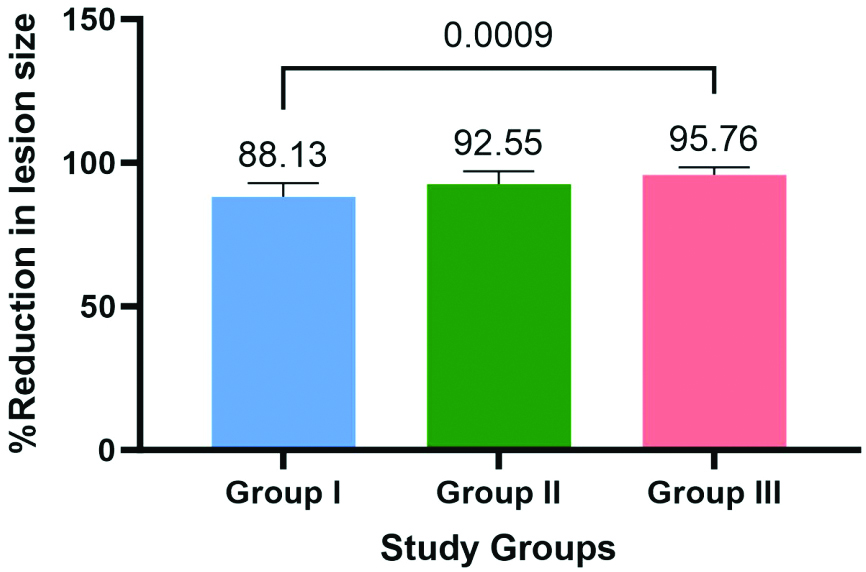
| % Reduction | 88.1±4.753 | 92.55±4.51 | 95.758±2.63 |
n: Sample size per study group; All values expressed as Mean (standard deviation)
The results of the One-way ANOVA assessing the percentage reduction of periapical lesion size among Group I, Group II and Group III, revealing a statistically significant difference (p<0.001) is shown in [Table/Fig-7,8]. Post-hoc Tukey’s tests further indicated significant differences between Group I and Group III (p<0.001).
Pair-wise comparisons for the decrease in lesion size (in mm2) by the post-hoc Tukey’s test.
| Comparison | p-value |
|---|
| Group I vs. Group II | 0.06NS |
| Group I vs. Group III | <0.001* |
| Group II vs. Group III | 0.20NS |
NS: Not statistically significant (p>0.05); *: statistically significant (p≤0.05)
Bar graph showing the percentage reduction in the lesion size (in mm2) for the study groups.
Intragroup comparison of bone density (HU) among the three groups showed the following results: In Group I, the mean±SD HU were 482.16±138.31 preoperatively and 1194.36±197.303 at the 12-month follow-up (p<0.001). In Group II, the mean±SD HU was 500.43±82.26 preoperatively and 1385.95±204.49 at the 12-month follow-up (p<0.001). Similarly, in Group III, the mean±SD HU was 478.26±164.05 preoperatively and 1371.82±241.75 at the 12-month follow-up (p<0.001) [Table/Fig-9].
Characteristics of the bone density units {Hounsfield Unit (HU)} (preoperative, postoperative and percentage gain) of all the study groups.
| Time points/Study groups | Group I(n=10) | Group II(n=10) | Group III(n=10) |
|---|
| Preoperative | 482.16±138.31 | 500.43±82.26 | 478.26±164.05 |
| Postoperative (12 months) | 1194.36±197.303 | 1385.95±204.49 | 1371.82±241.75 |
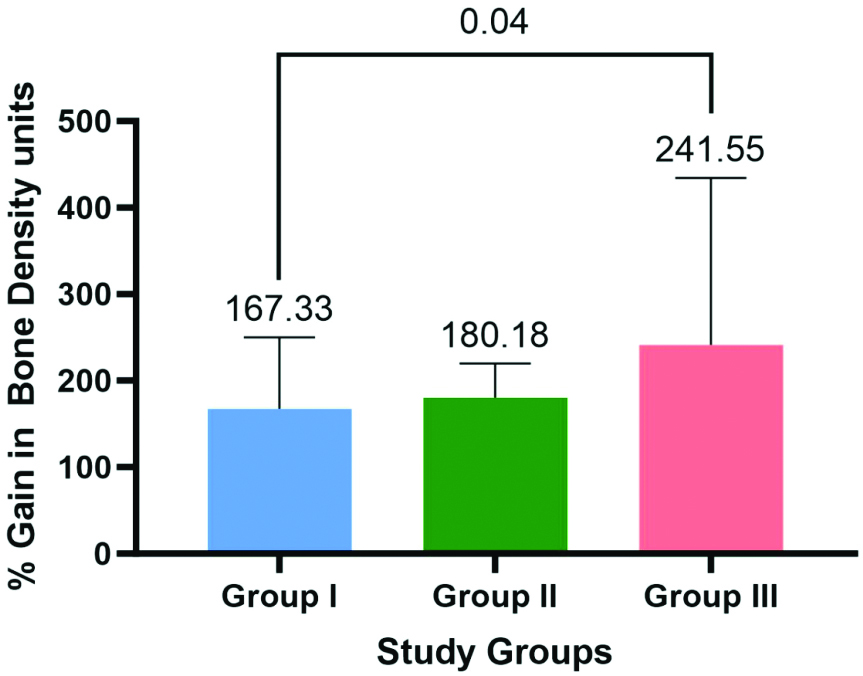
| % Gain | 167.33±82.904 | 180.18±39.406 | 241.55±192.734 |
n: Sample size per study group; All values expressed as mean (standard deviation); Postoperative bone density units significantly increased compared to preoperative measurements in all three groups (p<0.001)
Regarding percentage gain in bone density (HU), significant differences were observed between Groups-I, II and III based on one-way ANOVA (p=0.044). Subsequent Tukey’s post-hoc analysis identified a significant difference in % Gain between Group III and Group I (p=0.039) [Table/Fig-10,11].
Pair-wise comparisons for the increase in the bone density units {Hounsfield Unit (HU)} by the post-hoc Tukey’s test.
| Comparison | p-value |
|---|
| Group I vs. Group II | 0.69NS |
| Group I vs. Group III | 0.039* |
| Group II vs. Group III | 0.198NS |
NS: Not statistically significant (p>0.05); *: Statistically significant (p≤0.05)
Bar graph showing the percentage gain in the bone density units {Hounsfield Unit (HU)} for the study groups.
Discussion
The present study aimed to evaluate the effectiveness of different treatment modalities for periapical lesions, specifically comparing the outcomes of sticky bone combined with GTR membranes, sticky bone combined with PRF membranes and sticky bone alone. The findings of the present study revealed that both sticky bone combined with GTR membranes and sticky bone combined with PRF membranes resulted in significant bone healing and reduction in lesion size compared to the control group receiving only sticky bone. Among the experimental groups, the PRF membranes demonstrated superior outcomes, attributed to their bioactive properties that facilitate enhanced bone regeneration and soft-tissue healing.
Recent advancements in endodontic microsurgery have improved success rates to over 90% through the use of enhanced magnification, minimal root resection bevels, ultrasonic root-end preparation to depths of 3-4 mm and newer biocompatible root-end filling materials [15]. The goal of surgical endodontics is to achieve three-dimensional cleaning, shaping and obturation of the apical portion of the root canal system, which is inaccessible via a non surgical approach. Periapical surgery aims to remove periapical pathology and achieve complete wound healing through bone and periodontal tissue regeneration [16].
Studies support both surgical and non surgical approaches. Nair PR et al., emphasised that surgical intervention is essential for managing true periapical cysts due to their self-sustaining nature and resistance to non surgical root canal treatment [17]. These cysts often contain inflammatory cells and cholesterol crystals, which impede healing. In contrast, Ricucci D et al., suggested that some periapical cysts can heal after root canal treatment, with epithelial cells undergoing apoptosis and bone matrix forming around the lesion, indicating that cysts might delay but not prevent healing [18].
When surgical endodontic treatment is necessary, modern techniques produce better outcomes. The American Association of Endodontists (2010) and the Royal College of Surgeons of England (2012) favour microsurgical endodontic treatment, which removes pathological tissues and provides superior apical seals using materials like MTA. This material has properties conducive to bone healing, such as biocompatibility and a strong seal [19]. The present study followed similar protocols, selecting patients with periapical pathologies larger than 5 mm on intraoral radiographs. This size criterion was based on literature suggesting a higher incidence of cysts in lesions larger than 5 mm [20].
The study found that removing the apical 3 mm during root end resection eliminated most canal ramifications and accessory canals, crucial for a successful apical seal. Ultrasonic retro tips facilitated the preparation of retrocavities for effective sealing [21]. The use of bone grafts and barrier membranes, like sticky bone, GTR and PRF membranes, was also examined. Sticky bone, enriched with growth factors, proved effective in stabilising grafts and promoting bone regeneration [10].
The study’s results imply that integrating PRF membranes with sticky bone offers a more effective approach for managing periapical lesions than using sticky bone alone or in combination with GTR membranes. This is consistent with the observed faster bone regeneration and improved clinical outcomes in the PRF group. Previous studies have also supported these findings. For instance, Tsesis I et al., demonstrated that GTR techniques significantly improved periapical wound healing, especially in large lesions, aligning with the present study’s findings regarding the effectiveness of GTR membranes [22]. Similarly, Lin LM confirmed that GTR membranes are beneficial in endodontic surgery for improving healing outcomes in extensive periapical lesions. However, they also highlighted the limitations of GTR membranes as foreign bodies that may hinder natural healing processes, a concern noted in the present study as well [23].
In contrast, PRF membranes have been increasingly recognised for their advantages in regenerative procedures. Marx RE et al., established that PRF membranes enhance bone regeneration due to their autologous nature and ability to support cell migration and differentiation [24]. The findings of the present study corroborate this, showing that PRF membranes provide superior outcomes compared to GTR membranes. This aligns with Froum SJ et al., who emphasised the bioactive properties of PRF membranes in accelerating tissue repair and improving clinical results [25].
The present study employs advanced endodontic microsurgery techniques and novel materials like sticky bone, PRF membranes and GTR membranes to investigate their effectiveness in healing periapical lesions. Rigorous randomisation ensured unbiased group allocation and robust statistical power. Comprehensive CBCT scans enabled detailed evaluation of lesion size reduction and changes in bone density over 12 months.
Limitation(s)
However, despite these strengths, the study acknowledges several limitations, namely the variations in surgical techniques and patient responses may introduce confounding factors. Additionally, subjective radiographic assessments and a 12-month follow-up period may not adequately capture long-term outcomes or late complications. This suggesting a need for future multicentre studies with extended follow-up.
Conclusion(s)
In conclusion, the present study explored the effectiveness of sticky bone, PRF membranes and GTR membranes in enhancing healing outcomes following endodontic microsurgery for periapical lesions. Significant reductions in periapical lesion size and increases in bone density were observed across all treatment groups. Both the sticky bone combined with GTR membranes and sticky bone combined with PRF membranes resulted in significant bone healing and reduction in lesion size compared to the control group receiving only sticky bone. These results highlight the potential of advanced biomaterials in promoting tissue regeneration and improving clinical outcomes. However, given the study’s single-centre design and limited follow-up period, careful consideration is needed when interpreting these findings. Future multicentre trials with extended follow-up periods are essential to validate these results and to effectively refine surgical endodontic treatment protocols.
n: Sample size per study group; N: Total sample size; a: values expressed as Mean (standard deviation) and analysed by the One-way ANOVA test; b: values expressed in frequencies (percentage) and analysed by the Chi-square test; NS: Not statistically significant (p>0.05)
n: Sample size per study group; All values expressed as Mean (standard deviation)
NS: Not statistically significant (p>0.05); *: statistically significant (p≤0.05)
n: Sample size per study group; All values expressed as mean (standard deviation); Postoperative bone density units significantly increased compared to preoperative measurements in all three groups (p<0.001)
NS: Not statistically significant (p>0.05); *: Statistically significant (p≤0.05)