Breast carcinoma is the most common malignant tumour in women and the leading cause of mortality [1]. The aetiology of breast carcinoma includes advanced age, genetic factors, reproductive risk factors, and family history. Other factors positively associated with breast carcinoma are obesity, exogenous hormone use, radiation exposure, smoking, and alcohol consumption. Predisposing genetic risk factors include Breast Cancer Genes 1 and 2 (BRCA1, BRCA2), p53, and Checkpoint Kinase 2 mutation (CHEK2), which are the most critical genes responsible for increased breast cancer susceptibility [2]. Globally, about 1 million cases of breast carcinoma are diagnosed annually, with more than 170,000 are triple negative for ER, PR and HER2/neu (15%-20% of all breast carcinomas). TNBC is typically observed in young women who carry a mutation in the BRCA1 gene. It is characterised by a large mean tumour size, higher tumour grade, a higher rate of node positivity and, consequently, poor prognosis [3]. The diagnosis of TNBC is based on the assessment of Her2/neu status. Some cases that test positive for Her2/neu may require further detection via Fluorescence In Situ Hybridisation (FISH), which is more costly than IHC and increases the overall detection cost. Therefore, finding a more straightforward method for diagnosing TNBC has crucial clinical significance regarding the subsequent treatment of such patients [4].
One potential biomarker is fascin, which is usually expressed in neuronal, endothelial, and mesenchymal cells. Striking upregulation of fascin has been reported in several human malignant tumours, including breast, colon, pancreatic and lung carcinomas [5]. Fascin acts as an actin-binding protein that interacts with the actin cytoskeleton to induce migration, promoting metastasis and cell movement. FSCN1 (Fascin-1) is a gene responsible for an actin-bundling role that cross-links microfilaments into parallel rigid bundles, further facilitating metastasis [6]. High levels of cellular messenger Ribonucleic Acid (mRNA) for fascin enable the formation of migratory protrusions, thus promoting the migration and invasion of tumour cells [7]. Fascin is routinely used in clinics as a marker for staining Reed-Sternberg cells, which are present in classical Hodgkin’s lymphoma. Most IHC studies have shown that fascin expression is associated with the clinical aggressiveness of tumours and poor patient survival [8].
Thus, studying fascin and its role as a metastasis promoter is necessary to help develop novel therapeutic approaches targeting fascin. Present study was undertaken, since IHC is vital in accurately assessing fascin expression in breast carcinoma. The objective of the study was to determine the association of IHC expression of fascin with clinicopathological parameters in breast carcinoma.
Materials and Methods
The present hospital-based cross-sectional study was conducted in the Department of Pathology and Surgery at Pandit BD Sharma PGIMS, Rohtak, Haryana, India, from May 1, 2021, to April 30, 2022. This work was sanctioned by the Institutional Ethics Committee (IEC) of Pandit BD Sharma PGIMS, Rohtak, in 2021, under letter number BREC/Th/20/Patho/07 dated April 3, 2021.
Inclusion criteria: A total of 80 breast carcinoma specimens, including those from modified radical mastectomy and excisional biopsy, were included in the study.
Exclusion criteria: Breast malignancies other than carcinoma and inadequate biopsies were excluded from the study.
Study Procedure
The received specimens were fixed in neutral buffered formalin. As per standard protocol, representative microsections prepared from the blocks were stained with Haematoxylin and Eosin (H&E). IHC was performed using the biotin-avidin technique. After section cutting, the selected paraffin-embedded tissue blocks were placed on poly-L-lysine-coated slides for the IHC procedure. The primary antibodies used were anti-Fascin (Dako), ER (rabbit monoclonal ER α antibody, Dako), PR (rabbit monoclonal antibody, Dako), and Her2/neu (mouse monoclonal antibody, Biogenex). The expression of ER and PR was assessed using Allred scoring, which takes into account both the percentage and intensity of staining.
In each case, IHC fascin stains were interpreted subjectively by estimating the staining index, which was stratified into scores ranging from 0 to 12, depending on the proportion of stained tumour cells and the intensity [1]. Thus, fascin expression was determined by multiplying the proportion of stained tumour cells by the staining intensity, as shown in [Table/Fig-1]. Staining interpretation of fascin expression was classified as follows: Negative scores ranged from 0 to 3, while Positive scores ranged from 4 to 12 [1]. Fascin expression appeared as brown cytoplasmic staining in tumour cells. Positive and negative controls were run with each batch. The positive internal control was indicated by brown cytoplasmic staining in the stroma’s endothelial cells/myoepithelial cells. The negative control was obtained by substituting the primary antibody with a non specific reference antibody.
Scoring of the intensity and pattern of fascin staining.
| Score based on staining intensity | Staining intensity of malignant cells | Score based on percentage | Percentage- immune positive tumour cells |
|---|
| 0 | No staining | 0 | 0-5% |
| 1 | Mild staining | 1 | 6-25% |
| 2 | Moderate staining | 2 | 26-50% |
| 3 | Intense staining | 3 | 51-75% |
| 4 | >75% |
Statistical Analysis
The collected data were categorised, compiled, tabulated, and analysed using Statistical Package for Social Sciences (SPSS) version 24.0 software. All the data were enlisted, and an investigation proforma (including name, age, sex, clinical diagnosis, and history) was collected. Associations were tested using Pearson’s Chi-square and Fisher’s exact tests. A p-value <0.05 was considered as statistically significant.
Results
A total of 80 cases of breast carcinoma were included in the cross-sectional study, with patient age ranging from 28 to 82 years and a mean age of 51.5 years. The other clinicopathological details are summarised in [Table/Fig-2]. When segregating based on tumour size, the majority of the cases, 55 (68.75%), belonged to the subgroup of 2-5 cm, followed by groups over 5 cm, which comprised 16 (20%) of the cases. Infiltrating Ductal Carcinoma (IDC) - No Specific type (NOS) was the most common histologic subtype, accounting for 77.5%, followed by eleven cases of IDC with focal medullary-like features (13.75%). All cases were graded using Nottingham’s Bloom-Richardson grading system modification. Fifty percent of the total cases were classified as Grade II (moderately differentiated). Lymph nodes were involved in 37 (46.25%) cases, with 20 percent having 1-3 nodes. All cases were categorised based on tumour size, grade, and lymph node status. In most cases, 50 (62.5%) belonged to the moderate prognostic group. A significant statistical association was found (p-value=0.032) between fascin staining and age groups, with most patients aged 41-50 years. Fascin staining was significantly associated with tumour size (p-value=0.015), with the majority found in the size group of 2-5 cm. There was a statistical association between fascin cytoplasmic staining and the type of tumour (p-value=0.047), with most cases belonging to IDC-NOS. No association was found with histological grade, lymph node involvement or NPI category for prognostication. ER and PR expression were assessed using Allred scoring. Twelve (15%) cases were ER-positive, 18 (22.5%) cases were PR-positive, and 27 (33.75%) were positive for Her2/neu. [Table/Fig-3] shows a significant statistical inverse association between fascin staining and ER, PR, and Her2/neu, with a p-value of <0.001. Triple Negative/Basal-like was the most common molecular subtype, accounting for 53 out of 80 cases (66.25%). In the study, fascin cytoplasmic expression was noted in 50 (62.5%) of the cases. Further association with the molecular subtype was also significant, with most cases belonging to the TN/basal subtype, as shown in [Table/Fig-4]. The maximum number of cases (88.0%) positive for fascin were diagnosed as the TN/basal-like subtype, followed by the Her2/neu enriched subtype, which comprised eight percent of positive cases. Luminal A and B were the least common subtypes positive for fascin, each comprising two percent of the total positive cases. A significant statistical association was observed between fascin and molecular subtypes (p-value=0.051). [Table/Fig-5] shows a TN molecular subtype IDC exhibiting cytoplasmic fascin expression.
Association between fascin vs clinicopathological parameters.
| Parameters | | Fascin positive | Fascin negative | p-valve |
|---|
| Age (years) | 21-30 | 4 (8) | 0 | 0.032 |
| 31-40 | 10 (20.0) | 1 (3.33) |
| 41-50 | 13 (26.0) | 14 (46.6) |
| 51-60 | 12 (24.0) | 7 (23.3) |
| 61-70 | 9 (18) | 3 (10.0) |
| 71-80 | 2 (4.0) | 4 (13.3) |
| 81-90 | 0 | 1 (3.33) |
| Side | Right | 25 (50) | 18 (60) | 0.385 |
| Left | 25 (50) | 12 (40) |
| Tumor size (cm)- | <2 | 6 (12.0) | 3 (10.0) | 0.015 |
| 2-5 | 39 (78.0) | 16 (53.3) |
| >5 | 5 (10.0) | 11 (36.6%) |
| Histological subtype- | IDC-NOS | 42 (84.0) | 20 (66.6) | 0.047 |
| IDC+Medullary CA | 7 (14.0) | 4 (13.3) |
| Invasive lobular Carcinoma | 0 | 1 (1.25) |
| Invasive Mucinous CA | 0 | 3 (10) |
| Metaplastic CA | 1 (2) | 2 (6.6) |
| Histological grade | I | 10 (20.8) | 7 (23.3) | 0.891 |
| II | 26 (54.1) | 14 (46.7) |
| III | 14 (29.1) | 9 (30) |
| LN involvement- | 0 Nodes | 26 (52.0) | 17 (56.7) | 0.781 |
| 1-3 Nodes | 11 (22.0) | 5 (16.7) |
| 4-9 Nodes | 9 (18.0) | 4 (13.3) |
| ≥10 Nodes | 4 (8.0) | 4 (13.3) |
| NPI category | Good | 6 (12.0) | 7 (23.3) | 0.329 |
| Moderate | 34 (68.0) | 16 (53.3) |
| Poor | 10 (20.0) | 7 (23.3) |
LN: Lymph node; CA: Carcinoma; IDC: Infiltrating Ductal Carcinoma; NOS: No specific type; NPI: Nottingham prognostic index
Association of fascin expression with hormone receptor and Her2/neu.
| Parameters | Fascin n (%) | p-value |
|---|
| Positive (n=50) | Negative (n=30) |
|---|
| Oestrogen receptor | Positive | 2 (4.0) | 10 (33.3) | 0.001 |
| Negative | 48 (96.0) | 20 (66.7) |
| Progesterone receptor | Positive | 4 (8.0) | 14 (46.7) | 0.001 |
| Negative | 46 (92.0) | 16 (53.3) |
| HER2/neu | Positive | 9 (18.0) | 18 (60.0) | 0.001 |
| Negative | 41 (82.0) | 12 (40.0) |
Association between fascin and molecular subtype of breast carcinoma.
| Molecular subtype | Fascin n (%) | p-value |
|---|
| Positive | Negative | Total |
|---|
| Luminal A | 1 (2.0) | 3 (10) | 4 (5) | 0.051 |
| Luminal B | 1 (2.0) | 5 (16.7) | 6 (7.5) |
| HER2/neu enriched | 4 (8.0) | 13 (43.3) | 17 (21.25) |
| Triple negative/basal like | 44 (88.0) | 9 (30) | 53 (66.25) |
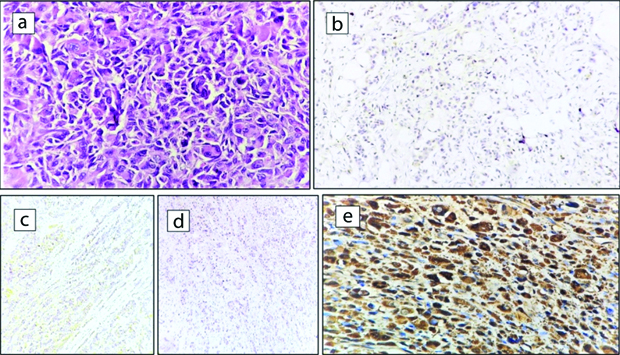
Case of Triple-Negative (TN)/basal-like breast carcinoma: a) IDC-NOS Grade III, (H&E 40x); b) ER Negative, (IHC, 4x); c) PR negative (IHC, 4x); d) Her2/neu Negative (IHC, 4x); e) Fascin positive (IHC, 40x).
Discussion
Breast cancer is the most common malignant tumour and the leading cause of death from carcinomas in the female population [1]. The most important prognostic factors in TNBC are the molecular subtype and protein receptor expression. Fascin is an actin-binding, motility-associated protein that is integral to the complex morphological changes and motility involved in metastasis [5]. Previous studies have shown a striking up-regulation of fascin in various malignancies, including breast carcinoma, demonstrating fascin positivity ranging from 16% to 58.53% [6-12]. In the current study, 62.5% of cases (50) showed positivity for fascin. It was found that most cases with cytoplasmic positivity for fascin was within the age group of 41-50 years, and a significant statistical association was observed between fascin and age (p-value=0.032). Similar to this study, Al Alwan M et al., and Yoder BJ et al., also found a significant association between age and fascin expression (p-values=0.043 and 0.049, respectively) [9,11]. In concordance with the research of Abbasi A et al., Yoder BJ et al., and Erdogan G et al., [10-12], IDC (NOS) constituted the largest group in this study (77.5%), with the maximum number of cases (84.0%) positive for fascin.
Fascin expression has been linked to the aggressive course of cancer cells through increased cell motility and augmented metastatic potential [5,7]. However, the current study found no association with tumour grade, with the maximum number of cases being grade II (50%). Lymph node involvement was assessed in all the cases, and staging was done based on the number of lymph nodes involved. In 53.75% of all cases, no lymph node involvement was observed. The results of Wang CQ et al., were similar to this study, with a maximum number of cases showing no nodal involvement [3].
In the literature, multiple studies [7,9-14] have found that fascin expression is inversely associated with hormonal receptors like ER and PR, with a maximum number of fascin-positive cases showing negative expression for both ER and PR. In line with these studies, the current study also found that ER and PR expression had an inverse relationship with fascin-positive cases, yielding a p-value of <0.001. Most fascin-positive cases were negative for ER (48/50, or 96%) and PR (46/50, or 92%).
Fascin overexpression has been linked to increased transcriptional activity of the fascin gene or degradation of the fascin protein, and is further related to HER2/neu gene amplification. In the study by Lee HJ et al., fascin overexpression was observed in HER2/neu-negative cases, and an inverse relationship was also shown [14]. Similarly, in the present research, most fascin-positive cases were negative for HER2/neu (41 cases out of 51 HER2/neu-negative cases, or 82%). This study observed high fascin overexpression in HER2/neu-negative cases, forming an inverse association with a p-value of 0.051. Alternatively, some studies did not show any association between fascin and HER2/neu status, such as those by Youssef NS and Hakim SA, Yoder BJ et al., and Erdogan G et al., [5,11,12].
In the current study, tumours were categorised into different prognostic groups according to NPI scoring system, which is based on tumour size, histologic grade, and lymph node status. A total of 62.50% (50/80) of all cases were in the moderate prognostic group, 21.25% (17/80) in the poor prognostic group, and 16.25% (13/80) in the good prognostic group. No association was found between these groups (p-value=0.329). Other studies do not mention the relationship between Fascin and the NPI scoring system, but they do note the poor prognosis associated with fascin-positive breast cancer. Erdogan G et al., mentioned that most of the IDC cases with positive lymph nodes were fascin-positive tumours [12]. Esnakula AK et al., depicted fascin expression in breast carcinoma as relatively more common in cases of disease recurrence (17/32) and distant metastasis (17/31), and therefore, debated Fascin’s critical role in epithelial-myoepithelial transformation, as well as further metastasis and poor prognosis [15]. None of the studies provided any association between NPI scoring and fascin expression.
Fascin has been observed to be linked to the TN/basal-like subtype [Table/Fig-6] [5,13-15]. Concise studies by Youssef NS et al., Min KW et al., Lee HJ et al., and Esnakula AK et al., [5,13-15] have shown a significant statistical association between fascin and molecular subtypes (p-value=0.051), with the highest percentage of cases (88.0%) positive for fascin belonging to the Triple Negative/basal-like subtype. Hormone-negative breast cancers traditionally have a poorer prognosis than hormone-positive cancers, as hormonal therapy is ineffective for them.
Association of Fascin with molecular subtypes in different studies.
| Study (year) | Fascin | Luminal A | Luminal B | HER 2 enriched | Triple negative | p-value |
|---|
| Youssef NS et al., (2014) [5] | Positive | 8 | 6 | 4 | 11 | 0.0007 |
| Negative | 18 | 14 | 5 | 1 |
| Esnakula AK et al., (2015) [15] | Positive | 18 | 2 | 7 | 55 | <0.0001 |
| Negative | 71 | 26 | 11 | 12 |
| Min KW et al., (2015) [13] | Positive | 0 | 5 | 11 | 7 | <0.05 |
| Negative | 2 | 28 | 15 | 1 |
| Lee HJ et al., (2017) [14] | Positive | 3 | 0 | 2 | 28 | <0.001 |
| Negative | 98 | 18 | 20 | 14 |
| Positive | 1 | 1 | 4 | 44 | <0.05 |
| Negative | 3 | 5 | 13 | 53 |
Limitation(s)
Due to the low sample size, further research on fascin expression in breast carcinoma is recommended on a larger scale, with follow-up and survival studies to validate the role of fascin in the aetiology or progression of breast cancer. This research will help in designing prognostic groups and treatment strategies.
Conclusion(s)
The study revealed a statistically significant association between fascin and the patient’s age, tumour size, histological subtype, ER, PR, HER2/neu status, and molecular subtype. There was a significant association between fascin expression and the TN subtype, with markedly increased intensity and extent of fascin expression (high score) compared to all other subtypes. This suggests that fascin is a marker for the TN/basal-like subtype and can be considered a promising candidate for targeted therapy in TNBC. The fencing antagonists have shown promising results in some studies with advanced TNBC that were fascin-positive.
Authors’ contribution: All the authors have contributed to the concept, literature search, data acquisition, data analysis, manuscript editing, and review.
LN: Lymph node; CA: Carcinoma; IDC: Infiltrating Ductal Carcinoma; NOS: No specific type; NPI: Nottingham prognostic index