Prognostic Implications of Modified Gleason Score and Gleason Grade Group in Histopathologic Study of Prostatic Adenocarcinoma: A Cross-sectional Study
Netra Prakash Kori1, Ranjana S Ranade2, Anuradha Patil2, Malashree2
1 Assistant Professor, Department of Pathology, SDM College of Medical Sciences and Hospital, Shri Dharmasthala Manjunatheshwara University, Sattur, Dharwad, Karnataka, India.
2 Associate Professor, Department of Pathology, KLE JGMM Medical College (A Unit of KAHER University, Belagavi), Gabbur Cross, Hubballi, Karnataka, India.
3 Associate Professor, Department of Pathology, MGM Medical College and Hospital, MGM University, Aurangabad, Maharashtra, India.
4 Assistant Professor, Department of Pathology, SDM College of Medical Sciences and Hospital, Shri Dharmasthala Manjunatheshwara University, Sattur, Dharwad, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ranjana S Ranade, Associate Professor, Department of Pathology, KLE JGMM Medical College (A Unit of KAHER University, Belagavi), Gabbur Cross, Hubballi-580028, Karnataka, India.
E-mail: ranjanaranade@gmail.com
Introduction
The Gleason Score (GS) is the most powerful prognostic indicator in prostatic carcinoma. The assignment of the GS is based on the histopathologic patterns of prostatic adenocarcinoma, which are classified according to the Gleason Pattern (GP). Periodic revisions in the definitions of the GP Score are attempted by the International Society of Urologic Pathology (ISUP), followed by the introduction of the Gleason Grade Group (GG) System, which aims to improve prognostication in prostate cancer.
Aim
To determine whether the ISUP 2014 GG group system is a better prognostic indicator compared to the ISUP 2005 Modified GS.
Materials and Methods
This cross-sectional study was conducted over four years, from January 2016 to December 2019, at the Department of Pathology, SDM College of Medical Sciences and Hospital, Shri Dharmasthala Manjunatheshwara University, Sattur, Dharwad, Karnataka, India. Core biopsies and transurethral resection biopsies of the prostate with a diagnosis of prostatic carcinoma were included in the study. Demographic details, Prostate-specific Antigen (PSA) levels and follow-up data were retrieved from the case files. Histopathology slides from all patients were examined and assigned GS scores from 6 to 10 and GG scores from 1 to 5. The slides were also analysed for the presence or absence of Perineural Invasion (PNI) and Cribriform Pattern (CFP). The percentage of tumour involvement was documented. Patients were followed-up for a period of two to five years for evidence of metastasis and/or death due to prostatic carcinoma. Categorical variables were analysed using descriptive statistics.
Results
The mean age of presentation was 70.21 years. A total of 52 cases were included in the study, with a histopathological diagnosis of adenocarcinoma in all cases. Three special variants were encountered, including one case each of Signet Ring Cell Carcinoma (SRCC), pseudo hyperplastic adenocarcinoma and intraductal foamy gland carcinoma. The distribution of GG scores was as follows: 7 (13.46%) for GG 1, 5 (9%) for GG 2, 6 (11.5%) for GG 3, 8 (15.38%) for GG 4 and 26 (50%) for GG 5. There were 23 cases with metastasis, with most belonging to GG 5 and GG 4. PNI and CFP were noted in 34 (65.38%) and 25 (48.07%) cases, respectively. Tissue cores with greater than 50% tumour involvement were observed in 32 cases (61.53%).
Conclusion
The current study underscores the prognostic importance of the ISUP 2014 GG system over the ISUP 2005 GS system. Histomorphological parameters such as PNI, CFP and a percentage of tumour involvement greater than 50% significantly influence prostate cancer prognosis. These factors may provide valuable information for optimal clinical management.
Cribriform pattern, Gleason grading, Prostate specific antigen, Prostatectomy
Introduction
Prostatic carcinoma is the second most common carcinoma affecting men and accounts for the fifth most common cause of cancer-related death [1]. In 1966, Donald Gleason first described a grading system for prostatic carcinoma based purely on histological architecture [2]. To this day, the GS remains the most powerful prognostic indicator in prostatic carcinoma. In the original system, grading was done on a scale of GP 1-5, depending on the architectural alterations observed in histology. The GS was determined by adding the two most common patterns present in the histological examination. However, the original system had many lacunae regarding clear-cut definitions and applicability. Assigning scores while evaluating multiple cores proved challenging and there were no clear descriptions of GP, specifically GP4. Additionally, there was no provision to report scores below 6. Furthermore, GS 7 included both GP 3+4 and 4+3, but these were found to be prognostically different [3].
To address these shortcomings, the ISUP made major revisions to the Gleason Grading System in 2005 and 2014. The recommendations from ISUP 2014 were included in the World Health Organisation (WHO) 2016 Classification of Prostatic Cancer [4] and are widely used in laboratory practice. In 2005, the assignment of GP 1 and 2 was withdrawn and GP 4 included the presence of cribriform and poorly formed glands. The Modified GS was defined in 2005 as the sum of the most common and the highest GP, instead of the first and second most common patterns [2,5]. In 2014, malignant prostatic glands with complex and non complex CFPs and/or glomeruloid patterns were assigned GP 4. The definitions of GP were refined and this system also introduced the GG group system, a five-tiered prognostic group for prostatic carcinoma, which was thought to be more patient-friendly than the 2005 GS system [6-9].
The key reason behind these revisions was to improve prognostication in prostate cancer. However, studies that have proven this point are scarce and most of them have correlated GG with Biochemical Recurrence (BCR) [10], rather than with clinical outcomes such as metastasis or death due to prostatic carcinoma. Very few studies worldwide have evaluated whether the ISUP 2014 revision predicts the prognosis of prostate cancer better than the ISUP 2005 GS system [11]. Additionally, the inclusion of histomorphological parameters such as PNI, CFP, Intraductal Carcinoma (IDC), the percentage of GP 4 and an overall measure of tumour extent may also serve as valuable prognostic indicators [12].
This study aimed to determine whether the ISUP 2014 GG system is a better prognostic indicator compared to the ISUP 2005 GS. Additionally, it seeked to further evaluate the role of other histopathological features, such as PNI, CFP, IDC, the percentage of core biopsy involved by GP 4 and an overall measure of tumour extent as prognostic parameters beyond what GS and GG can provide alone.
Materials and Methods
The present study is of cross-sectional type, conducted over a period of four years, from January 2015 to December 2019, in the Department of Pathology, SDM College of Medical Sciences and Hospital, Shri Dharmasthala Manjunatheshwara University, Sattur, Dharwad, Karnataka, India. After obtaining ethical clearance (Ref: SDMIEC/2023/571) from the Institutional Review Board, all biopsies diagnosed as prostatic adenocarcinoma were included in the study. Consent was not mandatory due to the retrospective nature of the study. To ensure confidentiality, participants’ histopathology and clinical data were linked to a unique code number.
Inclusion and Exclusion criteria: All biopsies {Transurethral Resection of Prostate (TURP)/core needle biopsy} diagnosed as prostatic adenocarcinoma were included in the study. After excluding inadequate and/or poorly preserved tissue samples, patients who had received prior chemotherapy, radiotherapy, or any other modality of treatment, as well as those who died due to other co-morbidities unrelated to prostatic carcinoma, a total of 52 cases were included in the study.
Study Procedure
The number of cores was counted for needle biopsy samples and all cores were submitted for histopathologic examination. The weight was measured in the case of TURP chips. If the total weight was <12 g, all chips were submitted for processing. One cassette was submitted for every additional 5 g of remaining prostatic chips. Demographic details, clinical presentations and preoperative and postoperative PSA levels were retrieved from hospital medical records.
The Haematoxylin and Eosin (H&E)-stained histopathology slides from these 52 patients were independently reviewed by two pathologists. GS was assigned for all the cases, while GG was assigned separately for each case according to the recommendations of the consensus meetings of ISUP in 2005 and 2014 [2,5]. According to the 2014 ISUP, cribriform, fused and glomeruloid glands were assigned to pattern 4 [Table/Fig-1]. GS of seven were further divided into two groups based on the proportion of patterns 3 and 4: 3+4=7 and 4+3=7. The patients were grouped into prognostic grade groups based on GS as follows: Group 1 (3+3); group 2 (GS 3+4); group 3 (GS 4+3); group 4 (GS 3+5; 4+4; 5+3); and group 5 (GS 5+4; 4+5; 5+5). Discrepancies in assigning GS and GG were resolved by consensus obtained through multiheaded microscope observation. The presence of PNI, IDC and CFP was documented. The percentage of tumour involvement (>50%) was noted separately for all cases. Cases with unusual histomorphological patterns were also observed, and GS and GG were assigned accordingly. Follow-up data regarding recurrence, treatment, metastasis and death were obtained from medical records and/or through telephonic communication. GG and GS were compared with the occurrence of metastasis and death.
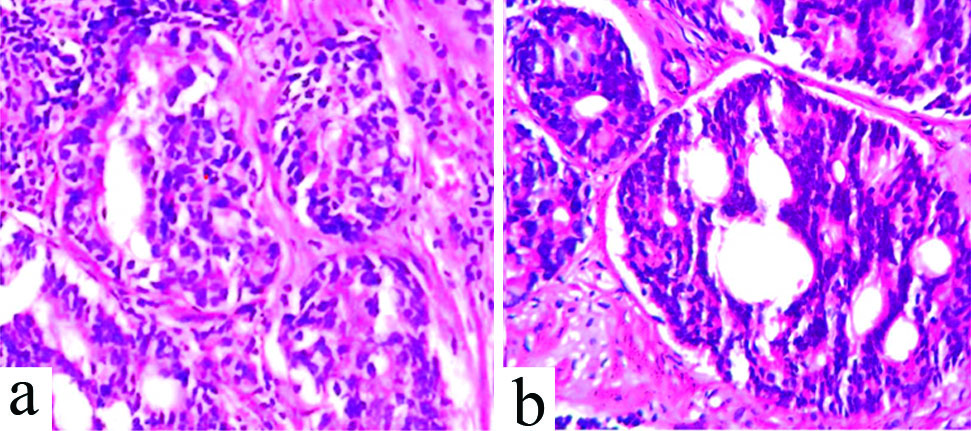
Assignment of Gleason Score (GS) according to ISUP 2005 and ISUP 2014 recommendations. a) Glomeruloid pattern (Fig.a, 400X, H&E) assigned Gleason Pattern (GP) 3 in ISUP 2005 recommendation was reassigned GP 4 as per ISUP 2014 recommendations. b) Small Cribriform Pattern (CFP) (Fig.b, 400X, H&E) was assigned Gleason Pattern (GP) 3 in ISUP 2005 recommendation, was reassigned GP 4 as per ISUP 2014 recommendations.
Statistical Analysis
Mean and Standard Deviation (SD) were calculated to describe continuous variables, while frequencies and percentages were used for categorical variables. Microsoft Excel was used for data entry and the Statistical Package for Social Sciences (SPSS) version 25.0 was used for descriptive statistical analysis.
Results
The present study had a total of 52 cases. The age distribution ranged from 52 to 88 years, with the mean age of presentation being 70.21 years. There were a total of 30 core needle biopsy specimens and 22 TURP specimens, with none being radical prostatectomy specimens. The histopathological diagnosis was adenocarcinoma in all the cases, which also included three unusual variants. The special variants were one case each of SRCC, pseudo-hyperplastic adenocarcinoma and intraductal foamy gland carcinoma [Table/Fig-2]. PSA levels were documented in all the cases during the preoperative and postoperative follow-up periods. Preoperative PSA levels ranged from 0.69 ng/mL to 1745 ng/mL, with an average of 173.48 ng/mL. All cases were classified according to ISUP 2005 and then regrouped according to the newer grading system as per the ISUP 2014 consensus. The grades of the cases studied in the current study were as follows: 7 (13.46%) in grade 1, 5 (9%) in grade 2, 6 (11.5%) in grade 3, 8 (15.38%) in grade 4 and 26 (50%) in grade 5, as shown in [Table/Fig-3]. The score was upgraded in 14 cases (26.92%) with the advent of the newer grading system. After re-evaluating with the revised score, seven cases were reassigned from group 4 to group 5. Three cases from group 2 were reallocated to group 5 and two cases from group 2 were moved to group 3. One case was reassigned from group 1 to group 4. There were 7 cases (13.46%) that were downgraded from the original scores to lower scores. In this, two cases were reassigned from group 5 to group 3, one from group 3 to group 2 and one from group 2 to group 1. One case was moved from group 4 to group 5. Follow-up details are tabulated and presented in [Table/Fig-4]. Metastatic deposits were observed in 23 (44.23%) cases in different parts of the body. The most common site was found to be the vertebrae (13 cases). Other sites of involvement included multiple bones (long bones, ribs, pelvic bones), the urinary bladder, liver and pelvic and paraaortic lymph nodes. The majority (14 cases) belonged to GG5 (10 cases) and GG4 (4 cases). The remaining cases belonged to GG3 (3 cases), GG2 (4 cases) and GG1 (2 cases). There were a total of 8 (15.38%) patients who died during the study period, with the majority of these (6 cases) being in GG5. Three of these patients also had metastasis. A comparison of GS (ISUP 2005) and GG (ISUP 2014) with the presence or absence of metastasis in these patients was tabulated [Table/Fig-5]. PNI was observed in 34 (65.38%) cases. The tumour involvement by tissue cores ranged from 20% to 85%. CFP was present in 25 (48.07%) cases. Tissue cores with over 50% tumour involvement were seen in 32 cases (61.53%). These histomorphology parameters were compared with the occurrence of metastasis [Table/Fig-6].
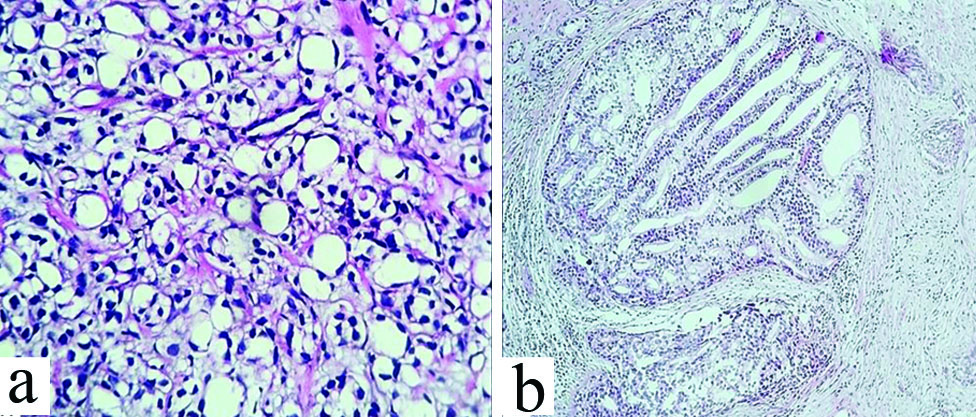
Unusual histopathologic variants of prostatic adenocarcinoma. a) Diffuse sheets of signet ring cells in case of signet ring cell adenocarcinoma (400x, H&E); b) Intraductal pseudohyperplastic tumour cells in glands, cords and Cribriform Pattern (CFP) (100x) in a case of pseudohyperplastic prostatic carcinoma.
Comparison of Gleason Scores (GS) as per ISUP 2005 and ISUP 2014 recommendations.
| Gleason Score (GS) (N=52) | ISUP 2005 | ISUP 2014 |
|---|
| 6 | 6 | 7 |
| 7 | 14 | 11 |
| 8 | 13 | 8 |
| 9 | 14 | 20 |
| 10 | 5 | 6 |
n=number of cases
Follow-up details of cases with prostatic adenocarcinoma.
| Parameters | Number of cases (N=52) | Percentage |
|---|
| Recurrence | 13 | 25% |
| Metastasis | 23 | 44.23% |
| Death | 8 | 15.38% |
| Prophylactic orchidectomy | 20 | 38.46% |
Comparison of GS and GG with trends of metastasis.
| GS ISUP 2005 | No. of cases (N=52) | Metastasis/death present n=23 cases n (%) | Metastasis/death absent n=29 cases n (%) | GG ISUP 2014 | No. of cases (N=52) | Metastasis/death present n=23 cases n (%) | Metastasis/death absent n=29 cases n (%) |
|---|
| 6 | 6 | 1 (16.66%) | 5 (83.33%) | 1 | 7 | 2 (28.57%) | 5 (71.42%) |
| 7 | 14 | 9 (64.28%) | 5 (35.71%) | 2 | 5 | 4 (80%) | 1 (20%) |
| 8 | 13 | 7 (53.84%) | 6 (46.15%) | 3 | 6 | 3 (50%) | 3 (50%) |
| 9 | 14 | 4 (28.57%) | 10 (71.42%) | 4 | 8 | 4 (50%) | 4 (50%) |
| 10 | 5 | 2 (40%) | 3 (60%) | 5 | 26 | 10 (38.4%) | 16 (61.53%) |
Comparison of histomorphological parameters with occurrence of metastasis.
| Histomorphologic pattern | Metastasis/death present n (%) | Metastasis/death absent n (%) |
|---|
| Presence of Perineural Invasion (PNI) (34 cases) | 24 (70.58%) | 10 (29.41%) |
| Cribriform Pattern (CFP) (38 cases) | 27 (71.05%) | 11 (28.94%) |
| Tumour involvement >50% (42 cases) | 18 (42.85%) | 24 (57.14%) |
Discussion
The incidence of prostate cancer varies across different regions of the world, representing 7.1% of all cancers in men [13]. Prostate cancer incidence rates are highly variable worldwide. Africa (26.6%) and Asia (11.5%) account for lower incidence rates compared to developed countries such as North America (73.7%), followed by Europe (62.1%). Age plays a significant role in the incidence and mortality rates of prostatic carcinoma, with almost 55% of all deaths occurring after 65 years of age [1]. The mean age of the patients in this study was 70 years. In a study by Ceyhan E et al., the mean age was found to be 63.1±6 years, which also included radical prostatectomy specimens [14]. The mean PSA value was 173.40 ng/mL, while a lower mean value of 14.8±6.7 was reported. The presence of a proportion of pattern 4 had a significant impact on assigning grades. The separation of GS 7 into 3+4=7 (GG2) and 4+3=7 (GG3) has different implications for management and treatment. For example, a prostatic core with a GS of 3+4=7, where pattern 4 accounted for <5%, behaved like 3+3=6 regarding BCR-free survival rate and had similar prostatectomy findings, thus qualifying it for active surveillance [9]. There were a total of 14 cases assigned GS 7 according to the 2005 ISUP guidelines. As per the 2014 ISUP guidelines, this was decreased to 11 cases (3+4=5 cases, GG2; 4+3=6 cases, GG3). Total of 66.66% (4 cases) with 4+3 and 60% of cases with 3+4 presented with metastasis. Even though this difference is not statistically significant due to a smaller sample size and limited duration of follow-up, it underscores the importance of segregating GS 7 into 3+4 and 4+3. GS of 10 was assigned to 5 cases according to ISUP 2005 and was outnumbered by 1 according to ISUP 2014. GG5 constituted 50% of cases, which included GP 4+5 (12 cases), 5+4 (8 cases) and 5+5 (6 cases). A 31.57% of the cases assigned with GS 9 and 10 presented with metastasis when compared to 38.4% of GG5 cases that had metastasis. The examination of radical prostatectomies, targeted biopsies with radiologic assistance, along with core needle biopsies, could better delineate the prognostic differences [15-18].
Authors observed that 70.58% of the present cases displayed PNI, 71% of cases with CAF were present and 42.85% of cases with more than 50% tumour involvement on histopathology presented with metastasis. The measurement of the extent of a tumour, such as the total length of cancer in millimeters and/or the percentage of the core involved by the tumour, serves as a potential prognostic factor in prostatic carcinoma [19,20]. In a study by Zelic R et al., the number of cores with more than or equal to 50% cancer independently predicted cancer death, regardless of the GG [10]. They also proposed that comedo necrosis is an independent prognostic factor. The presence of other histomorphological features such as CFP, PNI and the percentage of GP4 may aid in patient prognostication along with GG [19,21-25]. In a study examining the statistical correlation between PNI and PSA recurrence, disease-free survival rates were higher in patients without PNI (64% versus 24%) [25]. Out of the eight cases that died during the study period, six belonged to GG 9 and 10, indicating an association of higher GG with poor prognosis. The remaining two cases that died belonged to lower grades. In these cases, the confounding factors of other co-morbidities could not be ruled out. The authors encountered 23 patients (44.23%) with metastasis. The average age of metastasis was 67.63 years and only one case presented with metastasis at less than 55 years. Over 50% of patients (14 cases) belonged to GS 9 and 8, with two cases assigned GS 10. Bone was the most common site, with a predilection for the lumbar vertebra (13 cases) and pelvic bones. Multiple sites of bone involvement (long bones, ribs, pelvic bones) were seen in seven cases. Urinary bladder involvement was noted in two cases, while liver metastasis, as well as pelvic and para-aortic lymph node metastasis, was observed in one case. Similar findings were reported by Ondo CZ et al., [26]. SRCC of the prostate is a rare tumour first described in 1981, characterised by sheets of tumour cells displaying intracytoplasmic vacuoles (GS 5) with compressed, crescent-shaped, eccentrically placed nuclei [27,28]. This rare variant accounts for 2.5% of cases of prostate adenocarcinoma. The diagnosis of primary prostatic SRCC is challenging, as a complete work-up is needed to rule out metastasis from gastrointestinal origins. Correct diagnosis of pseudo-hyperplastic carcinoma may be challenging, as it mimics benign prostatic hyperplasia. Immunohistochemistry is essential for the correct diagnosis and GS should be assigned according to standard definitions [29]. Foamy gland carcinoma is a unique variant of prostatic adenocarcinoma, first described in 1996. It is characterised by a 3+3 pattern, displaying well-formed discrete glands with relatively bland nuclei, inconspicuous nucleoli and frequent intraluminal dense pink secretions [30]. In the present study, the authors observed a GS of 4+4 with higher grade presentation.
Limitation(s)
The present study has some limitations. The regrouping of prostate biopsies according to the 2005 ISUP guidelines was prone to observer bias, as the pathologists were already accustomed to the newer 2014 ISUP recommendations as part of their routine institutional reporting. The findings from core biopsies and TURP chips were not correlated with the radical prostatectomy specimens. Additionally, the limited sample size and duration of follow-up may have interfered with the study results. Implementing optimal biopsy techniques, using an adequate number of cores, incorporating radiologic assistance and evaluating the role of confounding factors would greatly enhance the strength of the present study.
Conclusion(s)
The current study underscores the prognostic importance of the ISUP 2014 GG system over the ISUP 2005 GS system. Rare histopathologic variants of prostatic adenocarcinomas pose diagnostic challenges in assigning the GS and establishing the correct diagnosis. Histomorphological parameters such as PNI, CFP and the percentage of tumour involvement (greater than 50% involvement) significantly influence prostate cancer prognosis. These factors may provide valuable information for optimal clinical management.
n=number of cases
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? No
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jun 29, 2024
Manual Googling: Aug 17, 2024
iThenticate Software: Oct 12, 2024 (9%)
[1]. Rawla P, Epidemiology of prostate cancerWorld Journal of Oncology [Internet] 2019 10(2):63-89.10.14740/wjon119131068988 [Google Scholar] [CrossRef] [PubMed]
[2]. Epstein JI, Allsbrook WC, Amin MB, Egevad LL, The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic CarcinomaThe American Journal of Surgical Pathology 2005 29(9):1228-42.10.1097/01.pas.0000173646.99337.b116096414 [Google Scholar] [CrossRef] [PubMed]
[3]. Shah MB, Raju K, Kumar GH, Revisiting Prostate Biopsy with 2014 ISUP Modified Gleason Score and Gleason Grade-a cross section studyBiomedical Research and Therapy 2018 5(12):2918-25.10.15419/bmrat.v5i12.511 [Google Scholar] [CrossRef]
[4]. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE, The 2016 WHO Classification of tumours of the urinary system and male genital organs-Part B: Prostate and bladder tumoursEuropean Urology 2016 70(1):106-19.10.1016/j.eururo.2016.02.02826996659 [Google Scholar] [CrossRef] [PubMed]
[5]. Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading CommitteeThe 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading SystemAm J Surg Pathol 2016 40(2):244-52.10.1097/PAS.000000000000053026492179 [Google Scholar] [CrossRef] [PubMed]
[6]. Epstein JI, Amin MB, Reuter VE, Humphrey PA, Contemporary Gleason grading of prostatic carcinoma: An update with discussion on practical issues to implement the 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinomaAm J Surg Pathol 2017 41(4):e1-e7.10.1097/PAS.000000000000082028177964 [Google Scholar] [CrossRef] [PubMed]
[7]. Kryvenko ON, Epstein JI, Prostate Cancer Grading: A decade after the 2005 modified gleason grading systemArchives of Pathology & Laboratory Medicine 2016 140(10):1140-52.10.5858/arpa.2015-0487-SA26756649 [Google Scholar] [CrossRef] [PubMed]
[8]. Delahunt B, Lamb D, Srigley JR, Murray J, Wilcox C, Samaratunga H, Gleason scoring: A comparison of classical and modified (International Society of Urological Pathology) criteria using nadir PSA as a clinical end pointPathology 2010 42(4):339-43.10.3109/0031302100378792420438406 [Google Scholar] [CrossRef] [PubMed]
[9]. Leapman MS, Cowan JE, Simko J, Roberge G, Stohr BA, Carroll PR, Application of a Prognostic Gleason grade grouping system to assess distant prostate cancer outcomesEuropean Urology 2017 71(5):750-59.10.1016/j.eururo.2016.11.03227940155 [Google Scholar] [CrossRef] [PubMed]
[10]. Zelic R, Giunchi F, Fridfeldt J, Carlsson J, Davidsson S, Lianas L, Prognostic utility of the gleason grading system revisions and histopathological factors beyond gleason gradeClinical Epidemiology [Internet] 2022 14:59-70.10.2147/CLEP.S33914035082531 [Google Scholar] [CrossRef] [PubMed]
[11]. Montironi R, Cheng L, Lopez-Beltran A, Scarpelli M, Mazzucchelli R, Mikuz G, Original Gleason System Versus 2005 ISUP Modified Gleason System: The importance of indicating which system is used in the patient’s pathology and clinical reportsEuropean Urology 2010 58(3):369-73.10.1016/j.eururo.2010.04.02820478652 [Google Scholar] [CrossRef] [PubMed]
[12]. Epstein JI, Prostate cancer grading: A decade after the 2005 modified systemModern Pathology 2018 31(S1):47-63.10.1038/modpathol.2017.13329297487 [Google Scholar] [CrossRef] [PubMed]
[13]. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countriesCA: A Cancer Journal for Clinicians [Internet] 2018 68(6):394-424.10.3322/caac.2149230207593 [Google Scholar] [CrossRef] [PubMed]
[14]. Ceyhan E, Yılmaz B, Öztürk B, The comparison of ISUP grades between prostate biopsy and radical prostatectomy: The incoherence and related factorsActa Medica 2021 52(3):213-18.10.32552/2021.ActaMedica.580 [Google Scholar] [CrossRef]
[15]. Connor MJ, Miah S, Jayadevan R, Khoo CC, Eldred-Evans D, Shah T, Value of systematic sampling in an mp-MRI targeted prostate biopsy strategyTranslational Andrology and Urology [Internet] 2020 9(3):1501-09.[Cited 2020 Oct 10]10.21037/tau.2019.07.1632676437 [Google Scholar] [CrossRef] [PubMed]
[16]. Ooi K, Samali R, Discrepancies in Gleason scoring of prostate biopsies and radical prostatectomy specimens and the effects of multiple needle biopsies on scoring accuracy. A regional experience in Tamworth, AustraliaANZ Journal of Surgery 2007 77(5):336-38.10.1111/j.1445-2197.2007.04054.x17497970 [Google Scholar] [CrossRef] [PubMed]
[17]. Dolatkhah S, Mirtalebi M, Daneshpajouhnejad P, Barahimi A, Mazdak H, Izadpanahi MH, Discrepancies between biopsy gleason score and radical prostatectomy specimen gleason score: An Iranian ExperienceUrol J 2019 16(1):56-61. [Google Scholar]
[18]. Coogan CL, Latchamsetty KC, Greenfield J, Corman JM, Lynch B, Porter CR, Increasing the number of biopsy cores improves the concordance of biopsy Gleason score to prostatectomy Gleason scoreBJU International 2005 96(3):324-27.10.1111/j.1464-410X.2005.05624.x16042723 [Google Scholar] [CrossRef] [PubMed]
[19]. Quintal MM, Meirelles LR, Freitas LL, Magna LA, Ferreira U, Billis A, Various morphometric measurements of cancer extent on needle prostatic biopsies: Which is predictive of pathologic stage and biochemical recurrence following radical prostatectomy?Int Urol Nephrol 2011 43(3):697-705.10.1007/s11255-011-9901-521340462 [Google Scholar] [CrossRef] [PubMed]
[20]. Rajab R, Fisher G, Kattan MW, Foster CS, Oliver T, Møller H, Measurements of cancer extent in a conservatively treated prostate cancer biopsy cohortVirchows Archiv 2010 457(5):547-53.10.1007/s00428-010-0971-z20827488 [Google Scholar] [CrossRef] [PubMed]
[21]. Harnden P, Shelley MD, Clements H, Coles B, Tyndale-Biscoe RS, Naylor B, The prognostic significance of perineural invasion in prostatic cancer biopsiesCancer 2007 109(1):13-24.10.1002/cncr.2238817123267 [Google Scholar] [CrossRef] [PubMed]
[22]. Ström P, Nordström T, Delahunt B, Samaratunga H, Grönberg H, Egevad L, Prognostic value of perineural invasion in prostate needle biopsies: A population-based study of patients treated by radical prostatectomyJournal of Clinical Pathology 2020 73(10):630-35.10.1136/jclinpath-2019-20630032034057 [Google Scholar] [CrossRef] [PubMed]
[23]. Leenders van, Kweldam CF, Hollemans E, Kümmerlin IP, Nieboer D, Verhoef EI, Improved prostate cancer biopsy grading by incorporation of invasive cribriform and intraductal carcinoma in the 2014 grade groupsEuropean Urology 2020 77(2):191-98.10.1016/j.eururo.2019.07.05131439369 [Google Scholar] [CrossRef] [PubMed]
[24]. Kweldam CF, Kümmerlin IP, Nieboer D, Verhoef EI, Steyerberg EW, van der Kwast TH, Disease-specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsyModern Pathology 2016 29(6):630-36.10.1038/modpathol.2016.4926939875 [Google Scholar] [CrossRef] [PubMed]
[25]. Kweldam CF, Kümmerlin IP, Nieboer D, Steyerberg EW, Bangma CH, Luca Incrocci, Presence of invasive cribriform or intraductal growth at biopsy outperforms percentage grade 4 in predicting outcome of Gleason score 3+4=7 prostate cancerModern Pathology 2017 30(8):1126-32.10.1038/modpathol.2017.2928530220 [Google Scholar] [CrossRef] [PubMed]
[26]. Ondo CZ, Ndiath A, Sarr A, Thiam A, Sine B, Sow O, Metastatic prostate cancer: Clinical aspects and treatment limitations in a university hospital center in SenegalAfrican Journal of Urology 2021 27(1):12110.1186/s12301-021-00223-0 [Google Scholar] [CrossRef]
[27]. Giltman LI, Signet ring adenocarcinoma of the prostateThe Journal of Urology 1981 126(1):134-35.10.1016/S0022-5347(17)54414-66265656 [Google Scholar] [CrossRef] [PubMed]
[28]. Guerin D, Hasan N, Keen CE, Signet ring cell differentiation in adenocarcinoma of the prostate: A study of five casesHistopathology 1993 22(4):367-71.10.1111/j.1365-2559.1993.tb00137.x8514280 [Google Scholar] [CrossRef] [PubMed]
[29]. Torbenson M, Dhir R, Nangia A, Becich MJ, Kapadia SB, Prostatic carcinoma with signet ring cells: A clinicopathologic and immunohistochemical analysis of 12 cases, with review of the literatureMod Pathol 1998 11(6):552-59. [Google Scholar]
[30]. Arista-Nasr J, Martinez-Benitez B, Valdes S, Hernández M, Bornstein-Quevedo L, Pseudohyperplastic prostatic adenocarcinoma in transurethral resections of the prostatePathology & Oncology Research 2003 9(4):232-35.10.1007/BF0289338314688829 [Google Scholar] [CrossRef] [PubMed]