Primary Cutaneous Mucinous Carcinoma: Case Report of a Rare Adnexal Tumour
PP Sathi1, Janaky Ramakrishnan2, PK Ramesh3
1 Professor, Department of Pathology, KMCT Medical College, Kozhikode, Kerala, India.
2 Assistant Professor, Department of Pathology, KMCT Medical College, Kozhikode, Kerala, India.
3 Professor, Department of Surgery, KMCT Medical College, Kozhikode, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Janaky Ramakrishnan, Assistant Professor, Department of Pathology, KMCT Medical College, Kozhikode-673602, Kerala, India.
E-mail: janakyram87@gmail.com
Primary cutaneous mucinous carcinoma is a rare malignant adnexal tumour of the skin, with the most common location being the periorbital region. Differentiating this tumour from cutaneous metastases and mucinous adenocarcinomas from other sites can be challenging, and due to its rarity, this tumour merits a more detailed study. The authors present a case of a 55-year-old male who presented with a slow-growing tumour located below the right earlobe. The lesion was clinically differentially diagnosed as either a lymph node or a soft tissue mass. An excisional biopsy of the mass revealed features of cutaneous mucinous carcinoma. The patient was clinically assessed, and the possibility of metastasis from other sites was ruled out. Hence, the present case lesion was diagnosed as primary mucinous carcinoma of the skin. The patient is doing well on follow-up, with no history of recurrence or other relevant issues.
Adenocarcinomas, Excisional biopsy, Metastases
Case Report
A 55-year-old male, a known case of hypertension not on any medication and with no other co-morbidities, presented with complaints of a slow-growing tumour below the right earlobe for the past year. Clinically, the lesion was given a differential diagnosis of either a lymph node or a soft-tissue mass. Ultrasound findings suggested a benign cystic lesion, possibly an epidermal inclusion cyst. Upon clinical examination, the lesion revealed a 2×2 cm subcutaneous swelling with firm consistency. The patient underwent excision biopsy, and the specimen was sent to the Histopathology Department of the Institution.
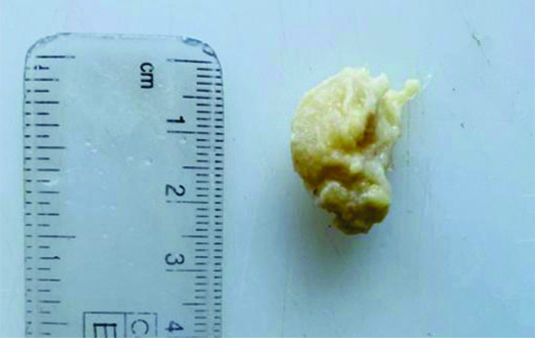
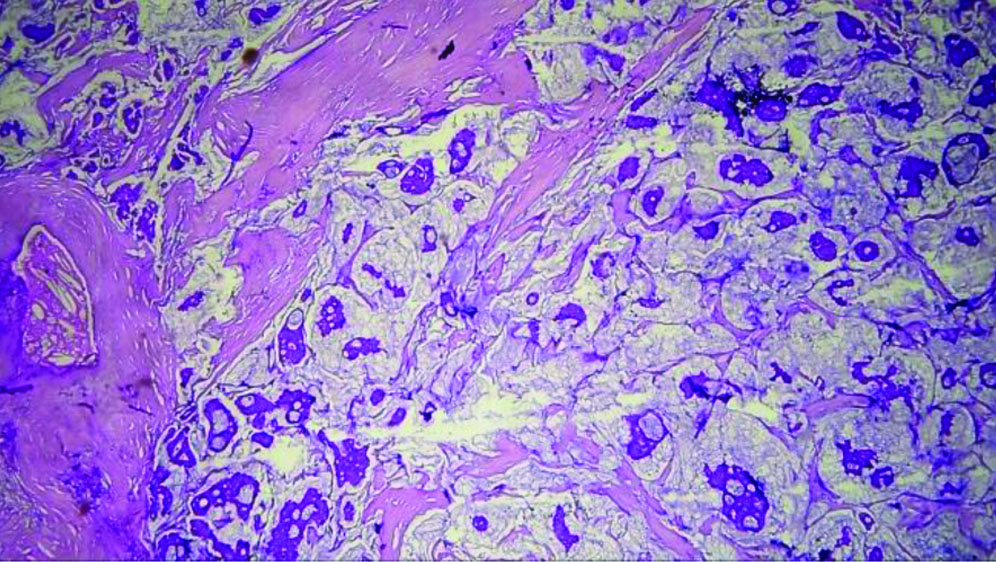
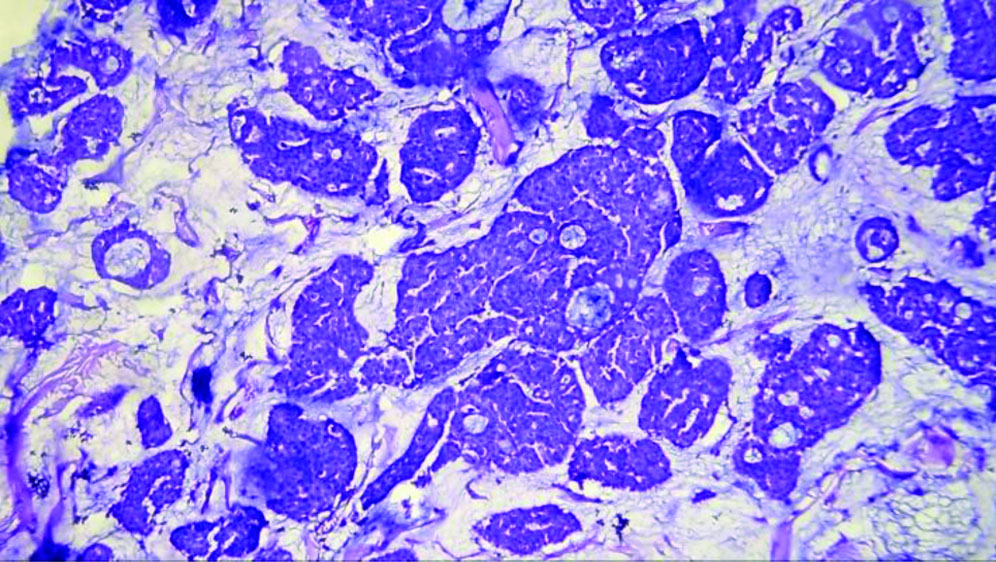
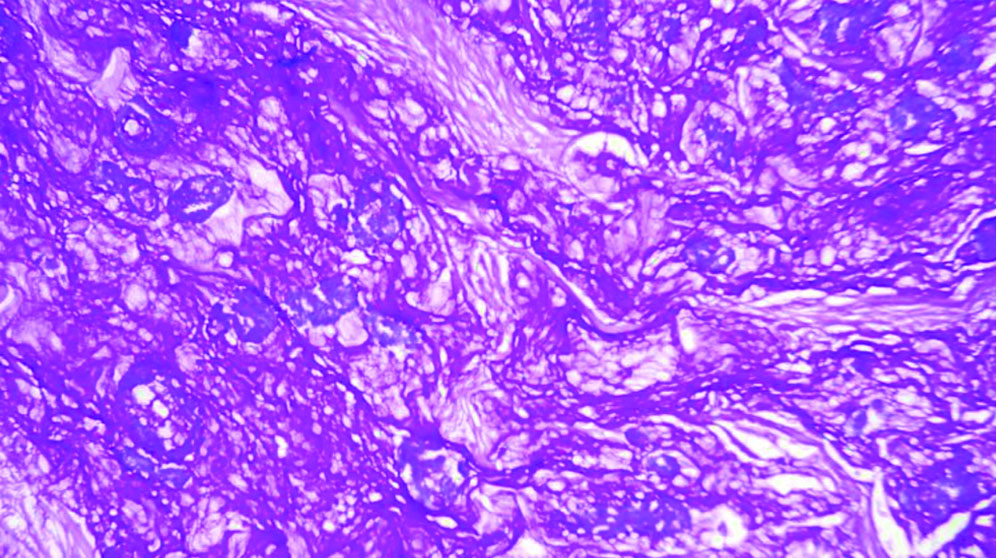
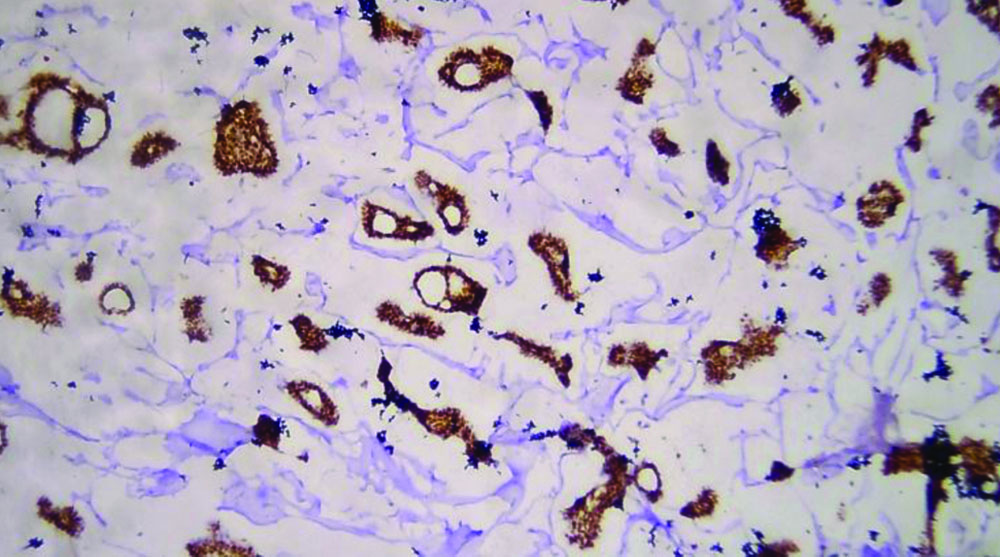
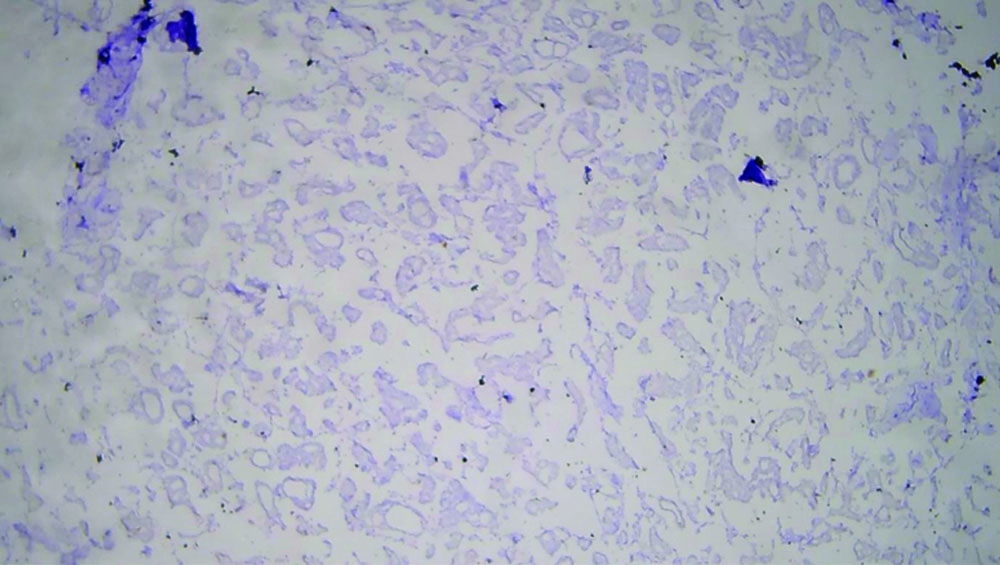
The specimen received in the department showed a pale white to translucent firm nodular mass measuring 2.5×2×1 cm. No attached skin was seen on gross examination. Cut sections revealed a solid, pale white gelatinous appearance [Table/Fig-1,2]. Formalin-fixed paraffin-embedded sections stained with Haematoxylin and Eosin (H&E) showed a fairly circumscribed neoplasm with tumour cells arranged in solid nests and glands floating in pools of extracellular mucin, separated by fibrocollagenous septae of varying thickness [Table/Fig-3]. Some areas in the periphery exhibited neoplastic cells arranged in closely packed glandular and cribriform clusters [Table/Fig-4]. No mitotic figures or necrosis were observed. The possible histopathological differential diagnoses included primary cutaneous mucinous carcinoma or a metastatic deposit from an unknown primary carcinoma. The extracellular mucin showed Periodic Acid-Schiff (PAS) positivity [Table/Fig-5]. Most cutaneous mucinous carcinomas are positive for oestrogen receptors and exhibit neuroendocrine differentiation. In the present case, Immunohistochemical (IHC) analysis revealed that tumour cells were positive for Oestrogen Receptors (ER) [Table/Fig-6] and negative for synaptophysin [Table/Fig-7].
Cut section; a solid, pale white gelatinous appearance.
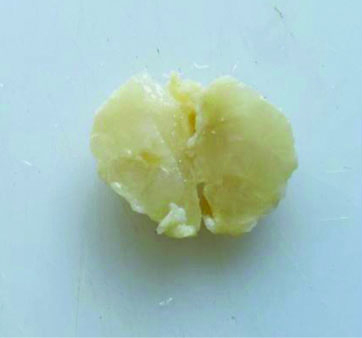
Gross appearance showing a nodular mass.
Tumour cells in solid nests and glands floating in pools of extracellular mucin, separated by fibrocollagenous septae (H&E, 10x).
Glands and cords of neoplastic cells floating in extracellular mucinous pools (H&E, 20x).
Extracellular mucin showing positivity (PAS, 10x).
Tumour cells showing nuclear positivity for oestrogen receptor (IHC, 20x).
Tumour cells negative for synaptophysin (IHC, 40x).
The case was discussed with the clinician, and further investigations, including radiography, ultrasound and computed tomography, were performed, all of which were negative. Thus, the possibility of metastasis from a mucinous adenocarcinoma from other sites was ruled out. Hence, a final diagnosis of primary mucinous carcinoma of the skin was reached.
Discussion
Primary mucinous carcinoma of the skin was first described by Lennox B et al., in 1952 [1]. It is a rare adnexal tumour with features similar to the mucinous lesions found in the breast. Some tumours that have a solid component with immunohistochemical features of neuroendocrine tumours are diagnosed as endocrine mucin-producing sweat gland carcinoma, which is regarded as a precursor lesion [2]. A review of 159 patients found that 77%, 13% and 10% of the patients were Caucasian, Asian and Black, respectively [3]. Both males and females are affected equally, with a predominant number of cases seen in the older population [4-6]. The most common location for this carcinoma is the face, particularly the periorbital region [3]. Rare cases have been reported in the anogenital area, supposedly arising from the mammary glands in that region [7]. Most cases of primary cutaneous mucinous carcinoma present as a single, bluish, flesh-coloured nodule or cyst that grows slowly. The mean diameter is reported to be approximately 1.8 cm [8].
Classic morphology shows nests, cords and singly scattered neoplastic cells floating in extracellular mucin pools, separated by fibrous septa of variable thickness. Primary cutaneous mucinous carcinoma shows a histopathological spectrum with two variants: a pure tumour where extracellular mucin comprises over 90% and a rare mixed form with an invasive ductal component [9]. Neoplastic cells are monomorphic with scant mitotic activity. An in situ component should be searched for, as it can be a supportive feature in defining the tumour as primary cutaneous. The in situ component can range from ductal and atypical ductal hyperplasia to ductal carcinoma in situ. Immunohistochemically, the neoplastic cells are positive for epithelial membrane antigen, low molecular weight cytokeratins, cadherins and gross cystic disease fluid protein-15. In most cases, neoplastic cells express oestrogen and progesterone receptors. Neuroendocrine differentiation may also be observed in primary cutaneous mucinous carcinoma. Although ultrastructural findings have proposed an eccrine origin for these tumours, about half of them show apocrine secretion, suggesting apocrine differentiation.
Primary cutaneous mucinous carcinoma is very rare. No morphological feature or immunohistochemical tool can clearly distinguish it from metastatic mucinous carcinoma. Primary cutaneous mucinous carcinoma has a better clinical outcome than metastatic lesions; therefore, differentiating between the two is crucial. Some features that can help differentiate primary lesions include their more organised neoplastic cells, lesser hyperchromasia, and low mitotic activity. In metastatic carcinoma, tumour cells invade between collagen bundles at the margins of the tumour.
The usual sites of origin for mucinous carcinoma include the breast, gastrointestinal tract, salivary glands, lacrimal glands, nose, paranasal sinuses, bronchi, renal pelvis and ovary [10]. Differentiating cutaneous mucinous carcinomas from breast carcinomas can be challenging. However, it is noteworthy that instances of breast mucinous carcinoma metastasising to the skin of the face or eyelid are very rare [11]. Staining for myoepithelial markers {tumour Protein 63 (p63) or Cytokeratin 5/6 (CK 5/6)} in the in situ component, if present, can help exclude metastatic mucinous breast carcinoma [12]. Metastasis from colorectal carcinoma can be excluded by immunohistochemical staining for CK20. In the present case, no primary tumour was detected in the breast, gastrointestinal tract, salivary glands, lungs, or kidneys.
The treatment for mucinous carcinoma is local excision. Due to an increased recurrence rate, excision with adequate margins is advised. Prognostically, tumours located in the head and neck have a much better outcome compared to those noted on the chest and back. A high recurrence rate and metastasis have been linked to younger age at presentation, white race and tumour size greater than 1.5 cm [3].
Since these tumours have a high recurrence rate, patients must be counselled about the need for frequent follow-ups for recurrence and regional lymphadenopathy. The present case patient is doing well nine months post-surgery during follow-up.
Conclusion(s)
Primary cutaneous mucinous carcinoma is a rare malignant adnexal carcinoma. The present case patient represents one of the few rare cases. Clinical distinction from metastatic mucinous carcinoma is of utmost importance. Clinicians should consider all possibilities before reaching a diagnosis, and investigations should be thoroughly reviewed to check for any signs of rare tumours.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jun 07, 2024
Manual Googling: Jul 12, 2024
iThenticate Software: Aug 19, 2024 (15%)
[1]. Lennox B, Pears AG, Richards HG, Mucin secreting tumors of the skin, with special reference to the so-called mixed salivary tumour of the skin and its relation to hidradenomaJ Pathol Bacteriol 1952 74:865-80.10.1002/path.170064041813000598 [Google Scholar] [CrossRef] [PubMed]
[2]. Freeman T, Russell AJ, Council ML, Primary cutaneous mucinous carcinoma: A review of the literatureDermatol Surg 2023 49(12):1091-95.10.1097/DSS.000000000000392137643246 [Google Scholar] [CrossRef] [PubMed]
[3]. Kamalpour L, Brindise RT, Nodzenski M, Bach DQ, Veledar E, Alam M, Primary cutaneous mucinous carcinoma: A systematic review and meta-analysis of outcomes after surgeryJAMA Dermatol 2014 150:380-84.10.1001/jamadermatol.2013.600624452370 [Google Scholar] [CrossRef] [PubMed]
[4]. Jhunjhunwala AK, Gharti Magar D, Upreti D, Thapa N, Ghosh A, Thapa S, Mucinous carcinoma of the skin: Acase reportJNMA J Nepal Med Assoc 2022 60:402-05.10.31729/jnma.741535633213 [Google Scholar] [CrossRef] [PubMed]
[5]. Papanastasiou AD, De Filippo MR, Sirinian C, Selenica P, Repanti M, Reis-Filho JS, Histologic and genomic characterization of a primary mucinous carcinoma of the skinEJC Skin Cancer 2023 1:10001110.1016/j.ejcskn.2023.10001138274496 [Google Scholar] [CrossRef] [PubMed]
[6]. Kim JW, Kim SE, Primary cutaneous mucinous carcinoma in a periorbital lesion: Two case reports and literature reviewArch Craniofac Surg 2024 25:90-94.10.7181/acfs.2024.0003138742336 [Google Scholar] [CrossRef] [PubMed]
[7]. Abiola OO, Ano-Edward GH, Oluwumi OA, Lasisi ME, Primary cutaneous mucinous carcinoma of the scrotum: A rare tumor at a rare site- A case report and review of literatureUrol Ann 2020 12(1):83-86.Available from: https://search.app/C6eg2ACNcnALiDbL910.4103/UA.UA_126_1832015625 [Google Scholar] [CrossRef] [PubMed]
[8]. Requena L, Kiryu H, Ackerman AB, Neoplasms with apocrine differentiation 1998 PhiladelphiaLippincott-Raven:907-47. [Google Scholar]
[9]. Yamamoto O, Nakavama K, Asahi M, Sweat gland carcinoma with mucinous and infiltrating duct-like patternsJ Cutan Pathol 1992 19:334-39.10.1111/j.1600-0560.1992.tb01371.x1331212 [Google Scholar] [CrossRef] [PubMed]
[10]. Tambe SA, Patil PD, Jerajani H, Recurrent primary mucinous carcinoma of the skinMedical Journal of Dr. D.Y. Patil Vidyapeeth 2023 16:S166-69.10.4103/mjdrdypu.mjdrdypu_103_21 [Google Scholar] [CrossRef]
[11]. Mukund T, Primary mucinous carcinoma of skin: A rare cutaneous neoplasm. Clinicopathologic features, differential diagnoses, and review of literatureAm J Dermatopathol 2024 46:114-20.10.1097/DAD.000000000000259138055969 [Google Scholar] [CrossRef] [PubMed]
[12]. Rakha E, Tan PH, Ellis I, Quinn C, Adenomyoepithelioma of the breast: A proposal for classificationHistopathology 2021 79:465-79.10.1111/his.1438033829532 [Google Scholar] [CrossRef] [PubMed]