Nasal Rhinosporidiosis Diagnosed by Cytology: A Case Report
Yogamaya Pattanayak1, Goutami Das Nayak2, Ranjan Kumar Mallick3, Gouranga Charan Prusty4, Lity Mohanty5
1 Senior Resident, Department of Pathology, SCB Medical College, Cuttack, Odisha, India.
2 Assistant Professor, Department of Pathology, SCB Medical College, Cuttack, Odisha, India.
3 Assistant Professor, Department of Pathology, SCB Medical College, Cuttack, Odisha, India.
4 Assistant Professor, Department of Pathology, SCB Medical College, Cuttack, Odisha, India.
5 Professor and Head, Department of Pathology, SCB Medical College, Cuttack, Odisha, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Goutami Das Nayak, Assistant Professor, Department of Pathology, SCB Medical College, Mangalabag, Cuttack-753007, Odisha, India.
E-mail: goutamidn12@rediffmail.com
Rhinosporidiosis is a chronic granulomatous inflammation of mucosal sites caused by the fungus Rhinosporidium seeberi. The most common site of involvement is the nasal mucosa, followed by the lips, palate, uvula, maxillary antrum, epiglottis, larynx, pharynx and trachea and bronchi. It usually presents as a polypoidal, reddish, friable, painless, pedunculated, hyperplastic soft-tissue mass in the nasal area, typically with an indolent and chronic progression. It can be confused clinically with other benign lesions such as inverted papillomas, primary sinonasal tuberculosis, angiofibromas and malignancies like nasopharyngeal carcinoma. Hence, accurate and final diagnosis is vital, which is achieved through histopathology. This is a case of rhinosporidiosis in a 44-year-old male patient who presented with a polypoidal nasal mass. Cytosmears revealed numerous endospores and few sporangia of Rhinosporidium seeberi, along with inflammatory cells and foreign body-type giant cells. Biopsy highlighted sporangia with endospores in different stages of maturation and thus confirmed the final diagnosis. Although cytology is a simple, preliminary, rapid and inexpensive method, histopathology is the gold standard for a definitive diagnosis. Surgical removal of the lesion with cauterisation of the attachment base is curative in the majority of cases. Recurrence is variable in endemic areas, especially in mucosal sites like the oropharynx and paranasal sinuses. Since there is inadequate literature on the cytodiagnosis of rhinosporidiosis, this case report highlights the significance and utility of cytology in the early diagnosis of nasal rhinosporidiosis.
Cytosmears, Fine needle aspiration cytology, Sporangia
Case Report
A 44-year-old male patient presented to the Ear, Nose and Throat (ENT) outpatient department with the chief complaints of a painless mass protruding from his left nostril and difficulty in breathing for the past two months. On examination, there was a polypoidal, erythematous mass measuring 2 cm in diameter, obstructing the left nasal cavity. There was no history of trauma, bleeding, or headache. The patient had no history of diabetes, hypertension, or any previous operative history. A clinical diagnosis of sinonasal polyp was considered.
General physical examination and systemic examination were normal. Complete blood counts, Erythrocyte Sedimentation Rate (ESR), blood urea, serum creatinine, and chest X-ray were all within normal limits. A Contrast-Enhanced Computed Tomography (CECT) scan of the nose and paranasal sinuses revealed multiple well-defined polypoidal lesions in the left maxillary sinus, nasal concha, and nasopharynx, likely indicating nasal and sinus polyps.
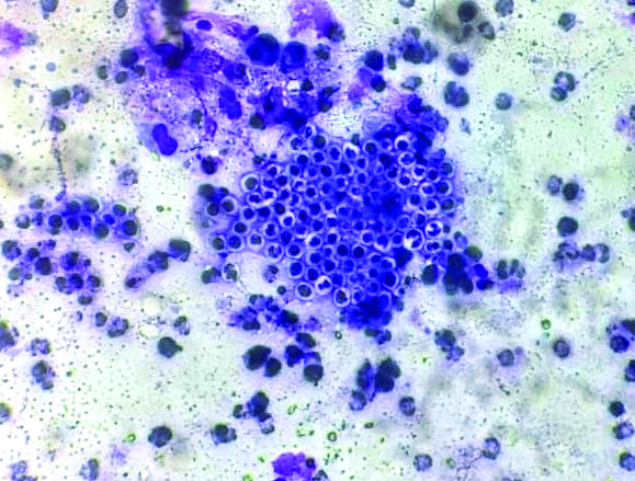
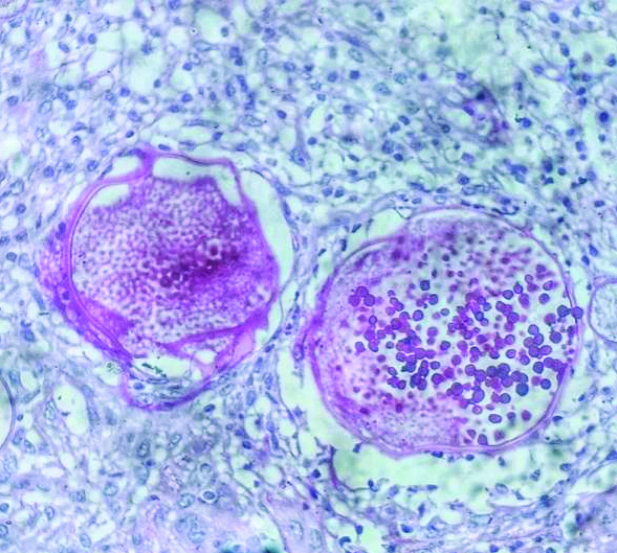
Fine Needle Aspiration Cytology (FNAC) from the lesion yielded blood-mixed thick necrotic aspirates. Ethanol-fixed smears were stained with Haematoxylin and Eosin (H&E) stains, while air-dried smears were stained with Diff-Quik stain. Microscopy of the smears showed numerous endospores [Table/Fig-1]. A few ruptured sporangia were also observed [Table/Fig-2]. The endospores were present in clusters and dispersed singly [Table/Fig-3]. They were spherical to oval, measuring 6-12 μm in diameter, had thick chitinous walls, and stained purple in the H&E stain. A mixed inflammatory infiltrate consisting of epithelioid histiocytes, lymphocytes, plasma cells and polymorphs was noted in the background [Table/Fig-4].
Cytosmear displaying Splendore- Hoeppli (H&E, 100x).
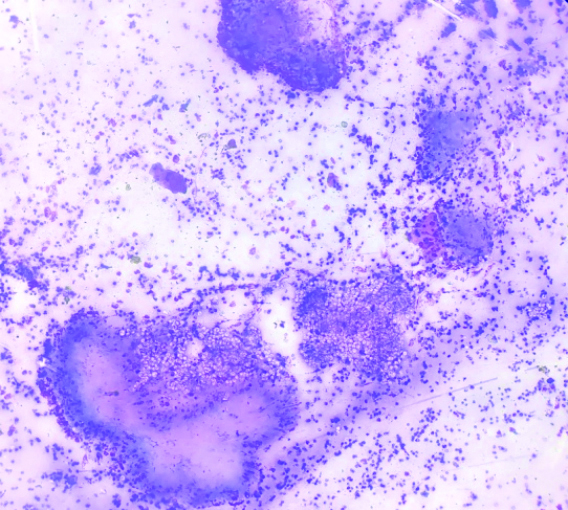
Cytosmear showing sporangia with endospores (Diff-Quik, 400x).
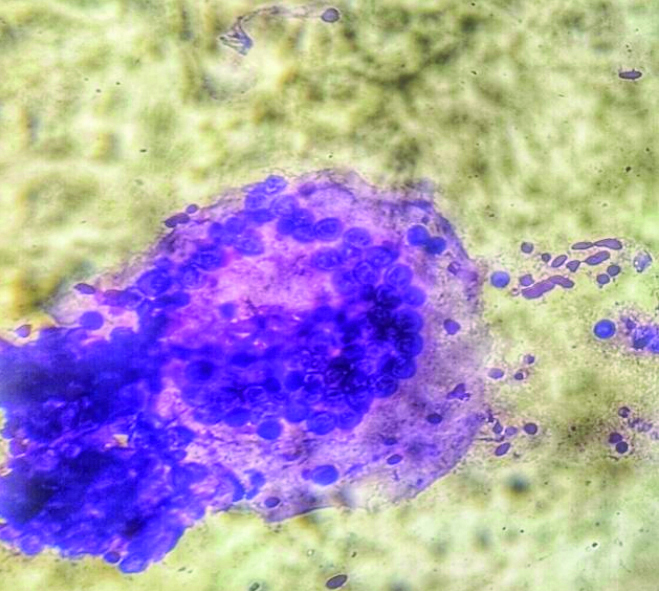
Cytosmear showing endospores and inflammatory cells (Diff-Quik, 400x).
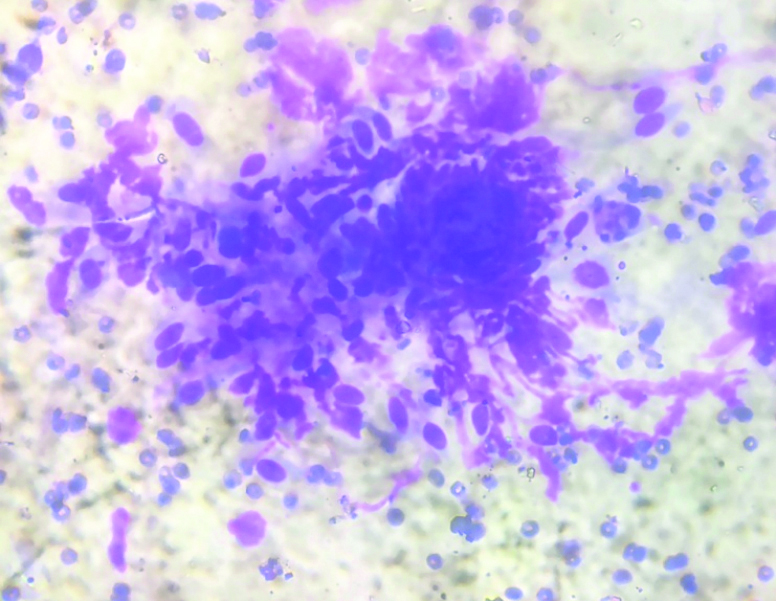
Cytosmear with epitheloid histiocytes, fibroblasts (H&E, 400x).
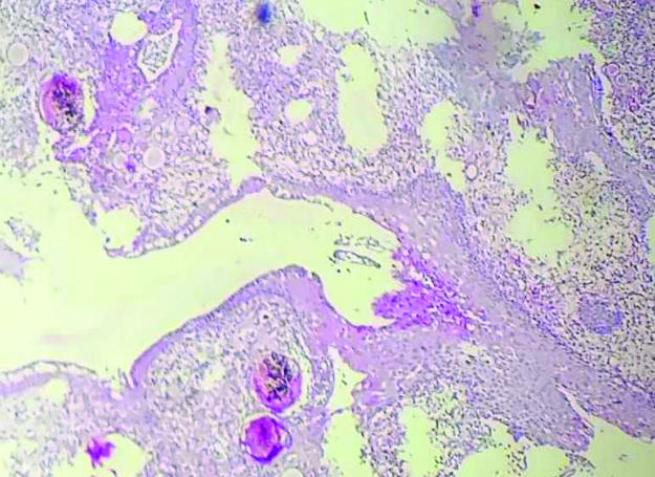
The lesion was surgically excised. Histopathology sections from the resected lesion revealed various sizes [Table/Fig-5] of intact and ruptured sporangia with endospores in the submucosal tissue of the nasal cavity [Table/Fig-6], surrounded by inflammatory infiltrates and a few foreign body giant cells.
Biopsy revealing nasal epithelium with sporangia in various stages of maturation containing spores (H&E, 100x).
Sporangia with endospores (H&E, 400x).
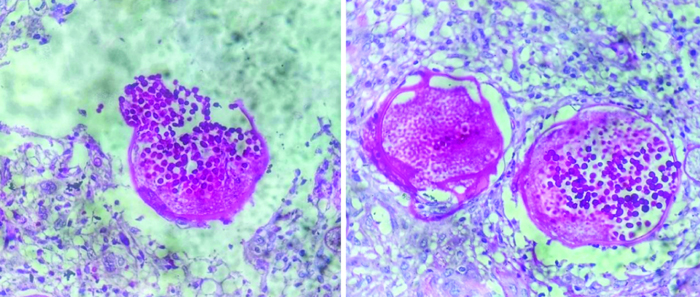
Periodic Acid-Schiff (PAS) stained histopathology sections showed deep magenta-stained walls of the endospores [Table/Fig-7]. Thus, the diagnosis of rhinosporidiosis was confirmed. Surgical removal of the lesion was performed with cauterisation of the attachment base. The patient was followed-up after six months and reported no further complaints.
Sporangia with endospores (PAS stain, 400x).
Discussion
Rhinosporidiosis is a chronic granulomatous inflammatory disease caused by the fungus Rhinosporidium seeberi, an aquatic protistan belonging to the group Mesomycetozoa [1,2]. The first cytological observation of rhinosporidiosis was made by Fortin and Meisels in 1974 [3]. It affects individuals of any age and sex in endemic areas such as India, Sri Lanka, South America, and Africa [4]. Typically, it is a localised condition characterised by polypoidal lesions in mucosal sites, including the nasal, oral, and genital areas [4]. Other rare sites of involvement include the conjunctiva, larynx, trachea, bronchi, skin, bone and viscera. The mode of infection occurs through air and water via traumatised epithelium, known as transepithelial infection [4]. Contact with contaminated water, dust, soil, clothing, and low socio-economic status are significant risk factors. There is evidence of haematogenous spread of rhinosporidiosis to anatomically distant sites [4,5]. Some rare disseminated forms of rhinosporidiosis have also been reported. Bansal R et al., reported a case of disseminated rhinosporidiosis presenting as multiple subcutaneous nodules in the ear, neck, thigh, supraclavicular region, chest wall and forearm [6]. Clinically, nasal rhinosporidiosis can be confused with benign neoplasms such as inverted papilloma, angiofibroma, and haemangioma, as well as malignancies like nasopharyngeal carcinoma.
The various developmental stages of sporangia in rhinosporidiosis can be readily identified using routine H&E staining and special fungal stains such as Gomori methenamine silver, PAS, and Mucicarmine stains [5,6]. Rhinosporidium seeberi has a morphology similar to Coccidioides, but its sporangia and endospores are larger than the spherules [7]. Both clinicians and pathologists should be aware of this condition when dealing with patients from endemic areas who present with nasal masses. Fine FNAC or scrape cytology of rhinosporidiosis is economical and reliable for preoperative diagnosis [7,8]. Rhinosporidiosis is typically managed through wide surgical excision with cauterisation of the base of the lesion to prevent recurrence [8]. Despite these measures, recurrence rates remain high, likely due to the spillage and seeding of sporangia during removal, which invade adjacent normal tissues [8,9]. Dapsone or antifungals have been tried in the past with variable success in selected cases, but with very high rates of recurrence due to the impenetrability of the sporangial wall to most antimicrobial agents. Furthermore, this organism is difficult to culture, making antibiotic susceptibility testing ineffective [9,10].
Conclusion(s)
Rhinosporidiosis is a chronic granulomatous inflammation characterised by transepithelial infection, primarily affecting the nasal mucosa. Both clinicians and pathologists should be aware of this condition and consider it in the differential diagnosis when dealing with patients from endemic areas who present with nasal masses. Fine FNAC or scrape cytology for rhinosporidiosis is rapid, economical, and reliable for preoperative diagnosis, leading to early detection and prompt management. Histopathology must be performed for confirmation, aided by special stains for definitive diagnosis. The main treatment modality is surgical excision with cauterisation of the base.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 05, 2024
Manual Googling: Sep 16, 2024
iThenticate Software: Sep 30, 2024 (17%)
[1]. Doddawad VG, Singh R, Shivananda S, A new technique to resolve nasal rhinosporidiosis: A case report with review of literatureInt J Surg Case Rep 2022 92:106807Epub 2022 Feb 4. PMCID: PMC885745710.1016/j.ijscr.2022.10680735180588 [Google Scholar] [CrossRef] [PubMed]
[2]. Deshpande AH, Agarwal S, Kelkar AA, Primary cutaneous rhinosporidiosis diagnosed on FNAC: A case report with review of literatureDiagn Cytopathol 2009 37(2):125-27.10.1002/dc.2096219021218 [Google Scholar] [CrossRef] [PubMed]
[3]. Fortin R, Meisels A, RhinosporidiosisActa Cytol 1974 18(2):170-73.4523819 [Google Scholar] [PubMed]
[4]. Bhargava S, Grover M, Maheshwari V, Rhinosporidiosis: Intraoperative cytological diagnosis in an unsuspected lesionCase Rep Pathol 2012 2012:101832Epub 2012 Oct 11. PMCID: PMC347753610.1155/2012/10183223097732 [Google Scholar] [CrossRef] [PubMed]
[5]. Arseculeratne SN, Recent advances in rhinosporidiosis and Rhinosporidium seeberiIndian J Med Microbiol 2002 20(3):119-31.10.1016/S0255-0857(21)03243-617657050 [Google Scholar] [CrossRef] [PubMed]
[6]. Bansal R, Gupta M, Jain V, Cytodiagnosis of disseminated rhinosporidiosis - A case reportInt J Tropical Disease & Health 2017 24(1):01-06.Available from: https://doi.org/10.9734/IJTDH/2017/3397810.9734/IJTDH/2017/33978 [Google Scholar] [CrossRef]
[7]. Arias AF, Romero SD, Garcés CG, Case report: Rhinosporidiosis literature reviewAm J Trop Med Hyg 2020 104(2):708-11.PMCID: PMC786636710.4269/ajtmh.20-029133289469 [Google Scholar] [CrossRef] [PubMed]
[8]. Pal S, Chakrabarti S, Biswas BK, Sinha R, Rakshit A, Das PC, Cytodiagnosis of extra-nasal rhinosporidiosis: A study of 16 cases from endemic areaJ Lab Physicians 2014 6(2):80-83.PMCID: PMC419636810.4103/0974-2727.14150125328331 [Google Scholar] [CrossRef] [PubMed]
[9]. Shenoy MM, Girisha BS, Bhandari SK, Peter R, Cutaneous rhinosporidiosisIndian J Dermatol Venereol Leprol 2007 73:179-81.10.4103/0378-6323.3274217558051 [Google Scholar] [CrossRef] [PubMed]
[10]. Bhat SP, Sajitha K, Shetty JK, Rhinosporidiosis- A retrospectionIndian J Pathol Oncol 2018 5(4):680-85.10.18231/2394-6792.2018.0130 [Google Scholar] [CrossRef]