Sepsis is a leading cause of morbidity and mortality worldwide, despite the existence of proper antimicrobial and palliative care. The World Health Organisation (WHO) 2018 report states that sepsis affects approximately 30 million people worldwide, potentially leading to six million deaths [1]. Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection [2]. The Third International Consensus Definitions of Sepsis and Septic Shock (2016) include the following criteria for sepsis [3]:
The SOFA score is used as a key criterion in the diagnosis of the sepsis syndrome at an individual patient level. There are 0-4 points allocated to each of the six organ systems (renal, cardiovascular, pulmonary, hepatic, neurologic, haematologic) in this 24-point assessment of organ dysfunction. Acute organ dysfunction is defined as an increase of two or more from baseline (if known) on the SOFA score [3]. Criteria for septic shock include sepsis plus the need for vasopressor therapy to elevate mean arterial pressure to >65 mmHg with a serum lactate concentration >2.0 mmol/L, despite adequate fluid resuscitation [3].
Sepsis starts with a source of infection in the skin, urinary tract, peritoneal cavity, lungs, and other areas of the body [4]. The body releases antigens as the organism multiplies, triggering a systemic inflammatory response that helps to eradicate and restrict the invasive virus. Furthermore, a variety of components of the invasive organism, such as beta-glucan from fungi, lipoarabinomannan from mycobacteria, endotoxins from gram-negative bacteria, and exotoxins from gram-positive bacteria, can also cause systemic inflammation [4]. As microbial components are identified by specific pattern recognition molecules (CD14 cells and Toll-like receptors), a complex process of cellular activation ensues, including the release of cytokines; activation of neutrophils, monocytes, and endothelial cells; neuroendocrine involvement; and activation of the complement, coagulation, and fibrinolytic systems. Monocytes and macrophages secrete Tumour Necrosis Factor (TNF) and interleukins 1 and 6, which trigger a cascade of inflammatory reactions [5]. When the local environment’s limitations are crossed by the production of proinflammatory mediators in reaction to an infection, a more widespread reaction takes place, leading to sepsis. In other words, sepsis is said to be a malignant intravascular inflammation [6].
Studies have shown that vitamin D is important for bone health and calcium homeostasis. Additionally, recent research indicates that vitamin D functions as an immune system mediator and has an inhibitory role in sepsis [7,8]. Antimicrobial Peptides (AMPs) are components of the innate immune system and have broad antibacterial, antiviral, and antifungal activity. Humans have two groups of AMPs: defensins and cathelicidins, and adequate concentrations of circulating 25(OH) vitamin D are required for optimal cathelicidin production by macrophages [9]. The adaptive immune system is also highly dependent on vitamin D. Over 100 genes have been identified as targets of 1,25(OH)2 vitamin D in mature T helper (Th) cells. Dendritic cells, which present antigens to T cells, are inhibited in their differentiation and activation by 1,25(OH)2 vitamin D. Vitamin D also affects the proliferation and differentiation of B cells and T cells and modulates immunoglobulin production [10,11]. Human endothelial cells treated with 1,25(OH)2 vitamin D and then stimulated with Lipopolysaccharide (LPS) demonstrated considerable suppression of molecules such as proinflammatory cytokines IL-6 and IL-8, as well as chemokines, when compared to cells solely exposed to LPS, according to a study by Equils O et al., [12]. Vitamin D could possibly play a role in the sepsis cascade triggered by fungi, as demonstrated by Khoo AL et al., who treated Peripheral Blood Mononuclear Cells (PBMCs) with 1,25(OH)2 vitamin D before exposing them to Candida albicans and found that the production of proinflammatory cytokines in PBMCs was significantly reduced in a dose-dependent manner, as was the expression of Pathogen Recognition Receptors (PRRs) that recognised Candida albicans [13]. There are only a few studies correlating serum vitamin D levels with the severity of sepsis [14,15]. Hence, this research work was carried out with an aim of assessing serum vitamin D levels in sepsis patients and studying the correlation between vitamin D levels with SOFA score as well as with the outcome of sepsis.
Materials and Methods
This was a cross-sectional study conducted in the Department of Medicine at Assam Medical College and Hospital, Dibrugarh, during the period from June 1, 2020, to May 31, 2021. The study was approved by the Ethics Committee of Assam Medical College and Hospital (IEC No. AMC/EC/PG/8898). Written informed consent was obtained from all study participants before enrolling them in the study.
Inclusion criteria: Patients included in the study were those aged over 18 years who met the Sepsis-3 clinical criteria, which include [3]:
A suspected infection
Acute organ dysfunction: defined as an increase of two or more points (from baseline, if known) on the SOFA score.
Exclusion criteria: Exclusion criteria included pregnant females; patients with a history of neuroendocrine diseases or mental disorders; consumption of corticosteroid drugs; recent and constant use of vitamin D; rickets; osteomalacia; and patients who did not provide consent.
Sample size: Taking a 95% confidence interval with an absolute error of 10%, and considering the proportion of patients with vitamin D deficiency to be 61.6% [16], the sample size was calculated and rounded off to be 91.
A detailed clinical history of the study subjects was taken, and a thorough general and systemic examination was performed. For the bedside diagnosis of sepsis, the qSOFA score was used, which has three components, each allocated one point [3]:
Respiratory rate ≥22/minute
Altered mentation
Systolic blood pressure ≤100 mmHg.
A score of ≥2 is associated with poor prognosis. This was followed by biochemical investigations, which included a complete haemogram, coagulation profile, serum creatinine, and liver function tests, all of which were utilised to calculate the SOFA score. Cultures of blood, urine, and sputum were obtained, and vitamin D levels were estimated.
SOFA scoring was done within 24 hours of admission and again after 72 hours. However, only the SOFA score obtained on admission (within 24 hours) was used to assess disease severity and to compare with vitamin D levels [Table/Fig-1,2] [17,18].
| System | 0 | 1 | 2 | 3 | 4 |
|---|
| Respiration PaO2/FiO2, mmHg | >400 | <400 | <300 | <200 with respiratory support | <100 with respiratory support |
| Coagulation Platelets ×103/μL | >150 | <150 | <100 | <50 | <20 |
| Liver Bilirubin (mg/dL) | <1.2 | 1.2-1.9 | 2.0-5.9 | 6.0-11.9 | >12.0 |
| Cardiovascular Mean Arterial Pressure (MAP) or administration of vasopressors (in μg/kg/min) required | MAP >70 mmHg | MAP <70 mmHg | Dopamine <5, or dobutamine (any dose) | Dopamine 5.1-15 or epinephrine <0.1 or norepinephrine <0.1 | Dopamine >15, or Epinephrine >0.1, or norepinephrine >0.1 |
| Central nervous system Glasgow Coma Scale | 15 | 13-14 | 10-12 | 6-9 | <6 |
| Renal Creatinine (mg/dL), or urine output (mL/day) | <1.2 | 1.2-1.9 | 2.0-3.4 | 3.5-4.9, or <500 mL/day | >5.0, or <200 mL/day |
Interpretation of the SOFA score [18].
| Maximum SOFA score | Mortality (%) |
|---|
| 0 to 6 | <10 |
| 7 to 9 | 15-20 |
| 10 to 12 | 40-50 |
| 13 to 14 | 50-60 |
| 15 | >80 |
| 15 to 24 | >90 |
Vitamin D assessment was performed using a competitive immunoassay on the VITROS 5600 fully automated integrated assay system. A review of the most recent literature [19] suggests the following recommendations for 25-OH vitamin D levels:
Deficient: <20 ng/mL (<50 nmol/L)
Insufficient: 20-29 ng/mL (50-75 nmol/L)
Sufficient: 30-100 ng/mL (75-250 nmol/L)
Potential toxicity: >100 ng/mL (>250 nmol/L)
Statistical Analysis
The statistical analysis of data was conducted using the computer programs Statistical Package for Social Sciences (SPSS for Windows, version 20.0 Chicago, SPSS Inc.) and Microsoft Excel 2010. Results for continuous measurements are presented as mean±standard deviation. Discrete data were analysed using the Chi-square test. Pearson’s correlation (r) was employed to measure the association among continuous variables. For all analyses, the statistical significance was set at a 5% level (p-value <0.05).
Results
The mean age of the study subjects was 57.34±16.55 years, with the maximum number of patients with sepsis (28.6%) being over 70 years of age. Out of 91 subjects, 49 (53.9%) were male, and 42 (46.1%) were female, resulting in a male-to-female ratio of 1.17:1.
Most cases were attributed to respiratory tract infections, followed in descending order by gastrointestinal diseases, neurological diseases, cardiovascular diseases, and urinary tract infections [Table/Fig-3].
Distribution of aetiology of sepsis.
| Disease/diagnosis | n (%) |
|---|
| Respiratory tract infections | 31 (34.1) |
| Gastrointestinal diseases | 21 (23.1) |
| Neurological diseases | 13 (14.3) |
| Cardiovascular diseases | 11 (12.1) |
| Urinary Tract Infection (UTI) | 10 (11.0) |
| Miscellaneous | 5 (5.5) |
Out of the 91 cases, 28 patients had positive body fluid cultures, while 63 had negative cultures. The highest percentage of positive cultures was observed in urine samples, accounting for 42.9% [Table/Fig-4].
Positive growth cultures from body fluids of study subjects.
| Culture | n (%) |
|---|
| Blood | 8 (28.6) |
| Urine | 12 (42.9) |
| Sputum | 5 (17.9) |
| Others (Peritoneal fluid, Pleural fluid, Cerebrospinal fluid) | 3 (10.7) |
| Total | 28 (100.0) |
Escherichia coli was the most common organism cultured 13 (46.4%), followed by Pneumococci 6 (21.4%), Pseudomonas 4 (14.3%), Klebsiella 3 (10.7%), and Entercoccus 2 (7.1%).
Most of the study subjects were vitamin D insufficient, 41 (45%), followed by those with vitamin D deficiency seen in 35 (38.5%). Only 15 (16.5%) of the subjects were vitamin D sufficient. Vitamin D deficiency increased with the age of the study subjects, with 48.6% of patients in the age group above 70 years being deficient in vitamin D. The relationship between vitamin D deficiency and age was statistically significant [Table/Fig-5].
Relationship of vitamin D level with age distribution.
| Age group (in years) | Vitamin D level (ng/mL) | p-value |
|---|
| Deficient (<20) | Insufficient (20-29) | Sufficient (30-100) |
|---|
| n (%) | n (%) | n (%) |
|---|
| 18-30 | 0 | 2 (4.9) | 5 (33.3) | <0.001 |
| 31-40 | 2 (5.7) | 6 (14.6) | 3 (20.1) |
| 41-50 | 1 (2.9) | 8 (19.5) | 2 (13.3) |
| 51-60 | 4 (11.4) | 8 (19.5) | 2 (13.3) |
| 61-70 | 11 (31.4) | 9 (22.0) | 2 (13.3) |
| >70 | 17 (48.6) | 8 (19.5) | 1 (6.7) |
| Total | 35 (100.0) | 41 (100.0) | 15 (100.0) | |
p-value calculated by Chi-square test The p-value is significant at 5% level of significance
There was a significant increase in the number of study subjects with deficient vitamin D levels, as well as vitamin D insufficiency, with increasing SOFA scores [Table/Fig-6]. The graph shows a negative correlation (Pearson coefficient, r=-0.420) between serum vitamin D levels and SOFA scores at 24 hours of admission, which was statistically significant (p-value <0.001) [Table/Fig-7].
Relationship of Vitamin D status with SOFA score.
| SOFA score | Vitamin D level (ng/mL) | p-value |
|---|
| Deficient | Insufficient | Sufficient |
|---|
| n (%) | n (%) | n (%) |
|---|
| 0-6 | 1 (2.9) | 17 (41.5) | 5 (33.3) | <0.001 |
| 7-9 | 4 (11.4) | 13 (31.7) | 4 (26.6) |
| 10-12 | 16 (45.7) | 8 (19.5) | 5 (33.3) |
| 13-14 | 5 (14.2) | 3 (7.31) | 1 (6.66) |
| ≥15 | 9 (25.7) | 0 | 0 |
Correlation between vitamin D levels and SOFA score.
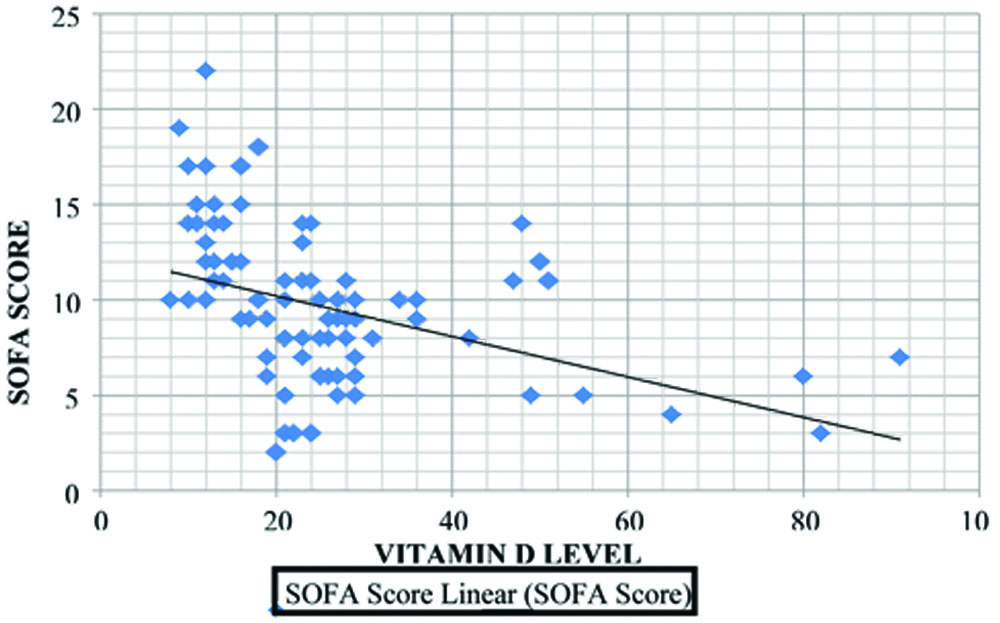
The association between vitamin D levels and the outcome of sepsis was statistically significant (p-value <0.001). The mean SOFA score was higher in non survivors than in survivors [Table/Fig-8].
Relationship of vitamin D level with outcome of sepsis.
| Outcome | Vitamin D level (ng/mL) | p-value |
|---|
| Deficient (<20) | Insufficient (20-30) | Sufficient (30-100) |
|---|
| n (%) | SOFA score (Mean±SD) | n (%) | SOFA score (Mean±SD) | n (%) | SOFA score (Mean±SD) |
|---|
| Survivor | 12 (34.3) | 9.31±4.02 | 30 (73.2) | 6.29±3.32 | 14 (93.3) | 7.96±3.79 | <0.001 |
| Non survivor | 23 (65.7) | 16.67±2.78 | 11 (26.8) | 11.7±1.41 | 2 (6.7) | 13.5±0.79 |
| Total | 35 (100.0) | | 41 (100.0) | | 15 (100.0) | | |
p-value calculated using Chi-square Test The p-value is significant at 5% level of significance
Discussion
The present study included 91 subjects and showed that sepsis affected individuals of all ages but was most common in the elderly population, particularly those above 70 years (28.6%). Males were more frequently affected, with a male-to-female ratio of 1.71:1. This was comparable to studies conducted by Angus DC et al., and Martin GS et al., who found a higher incidence of sepsis in older age groups [20,21]. A male preponderance was also observed by Martin GS et al., (53.9%) and Azim A et al., (61.39%) [21,22].
In present study, respiratory tract infections were the most common cause of sepsis (34.1%), followed by gastrointestinal diseases (23.1%) and urinary tract infections (11%). Sudhir U et al., found respiratory tract infections to be the aetiology of sepsis in 40% of cases, followed by urinary tract infections at 19% and gastrointestinal diseases at 8% [23].
This study indicated that out of the 91 subjects, 38.5% had deficient vitamin D levels, 45.1% had insufficient vitamin D levels, and 16.5% had sufficient vitamin D levels. The mean vitamin D level was 25.43±15.46 ng/mL. A negative correlation between serum vitamin D levels and SOFA scores was found within 24 hours after admission, which was statistically significant (p-value <0.001). A study by Jeng et al., showed that vitamin D insufficiency was present in 100% of critically ill patients with sepsis, 92% of critically ill patients without sepsis, and 16.5% in healthy controls [24]. However, they found a higher rate of 61.6% cases of vitamin D deficiency. A study conducted by Ginde AA et al., in 81 patients suspected of having an infection in the Emergency Department found that patients with serum 25 (OH)D concentrations less than 30 ng/mL were more likely to have severe sepsis and SOFA scores >2, and were more likely to have severe sepsis at 24 hours [25].
Among the patients with vitamin D deficiency, 45.7% had SOFA scores between 10-12, 14.2% had SOFA scores between 13-14, and 25.7% had SOFA scores >15. There was a significant increase in deficient vitamin D levels when the SOFA score was above 10, and insufficient vitamin D levels when the SOFA score was >4. The majority of patients with sufficient vitamin D levels had lower severity of sepsis, with SOFA scores ranging from 0 to 10. Findings similar to those of present study were reported by Seedat F et al., where critically ill patients with 25-hydroxyvitamin D levels <12 ng/mL exhibited increased organ system dysfunction and a greater change in SOFA scores [26]. Alves FS et al., evaluated the serum concentrations of vitamin D in patients with severe sepsis or septic shock and found that vitamin D concentrations <30 ng/mL (defined as insufficient levels) were present in 98% of cases [27].
In present study, a mortality rate of 38.5% was observed. Among the patients who expired, 65.7% were deficient in vitamin D, 26.8% were insufficient in vitamin D, and only a small fraction had sufficient vitamin D levels (6.7%). There was a statistically significant relationship between vitamin D levels and the outcome of sepsis (p-value <0.001) in this study. The outcome data indicated that the prognosis for patients with vitamin D deficiency and sepsis is poor, while those with sufficient vitamin D levels have a relatively better outcome. A study by Jeng et al., showed that vitamin D insufficiency was present in 100% of critically ill patients with sepsis, 92% of critically ill patients without sepsis, and 16.5% in healthy controls [24].
Yang B et al., conducted a retrospective cohort study in which critically ill patients admitted to intensive care units were divided into a vitamin D supplementation group and a non vitamin D supplementation group [28]. With an Odds Ratio (OR) of 0.70, multivariate regression analysis suggested that vitamin D supplementation may serve as a protective factor against in-hospital death. Patients with sepsis who received vitamin D supplementation exhibited significantly lower in-hospital 28-day and 90-day mortality rates.
Limitation(s)
The study was conducted at a single centre with a relatively small study population.
Conclusion(s)
This study has shown a high prevalence of Vitamin D deficiency and insufficiency in patients with sepsis, particularly among the elderly group. Patients with higher SOFA scores (beyond 10) exhibited a greater incidence of Vitamin D deficiency. This negative correlation was statistically significant. Additionally, lower levels of Vitamin D were associated with higher mortality rates and poorer outcomes.
p-value calculated by Chi-square test The p-value is significant at 5% level of significance
p-value calculated using Chi-square Test The p-value is significant at 5% level of significance