Thrombocytopenia, characterised by a platelet count below 150,000/μL, affects 7-11% of pregnancies for various reasons. Immune Thrombocytopenia (ITP), with platelet counts under 100,000/μL, accounts for 1-4% of pregnancy-related thrombocytopenia cases, particularly in the first and second trimesters. Not all pregnant patients with ITP require treatment, but when necessary, corticosteroids and Intravenous Immunoglobulins (IVIGs) are commonly used. This case reports a 25-year-old pregnant female, G3A2, at 27 weeks of gestation with a history of ITP and hypothyroidism. She presented with severe thrombocytopenia (8,000/μL) and a history of petechiae and gum bleeding. Initial treatment included corticosteroids, IVIG, and platelet transfusions, leading to fluctuating platelet counts. Due to refractory ITP, advanced treatments such as rituximab and romiplostim were administered. Despite significant challenges, including an episode of epistaxis and the need for intensive monitoring, the patient delivered a healthy baby via normal vaginal delivery at 35 weeks. The newborn required brief respiratory support and phototherapy for jaundice but had stable platelet counts at discharge. This case highlights the complexity of managing refractory ITP during pregnancy, emphasising the need for a multidisciplinary approach and individualised treatment plans to ensure maternal and foetal wellbeing. Continuous vigilance, adaptive strategies, and the use of multidrug therapy were crucial in achieving a successful outcome, underscoring the importance of tailored protocols and emerging therapies in such high-risk pregnancies.
Case Report
A 25-year-old pregnant female, G3A2, at 27 weeks of gestation, known to have ITP for three years and hypothyroidism, presented for further evaluation and management of thrombocytopenia (8×103/μL) in the haematology OPD. She denied any history of abdominal pain, vaginal bleeding, dysuria, or fever. The patient reported occasional petechiae on her upper and lower limbs and bleeding from her gums while brushing for the past three years, leading to a diagnosis of ITP. She has been intermittently taking tab Wysolone 50 mg since 2020, insulin for overt DM, and tab Thyronorm 25 mcg for hypothyroidism. She has a family history of Type 2 Diabetes Mellitus (T2DM) in both her mother and father.
She experienced menarche at the age of 12 years and has had regular menstrual cycles with no dysmenorrhea or menorrhagia. Married for five years, she has experienced two spontaneous abortions, both at five weeks of gestation, without further evaluation of the cause.
On examination, the patient was conscious and oriented with no pallor, icterus, clubbing, cyanosis, lymphadenopathy, or oedema. Petechiae were present on both upper limbs. Heart rate, blood pressure, and O2 saturation were within normal limits. The abdomen was longitudinally distended, and the uterus corresponded to 28 weeks of gestation upon performing an abdominal examination. Foetal movement and foetal heart sounds were present. All other systems were within normal limits. The patient was admitted, and routine laboratory investigations were sent [Table/Fig-1a].
Investigations of the patient.
| Parameters | Results | Reference value |
|---|
| Haemoglobin (g/dL) | 10.3 | 12-14 |
| Total WBC count (×103/μL) | 9.48 | 4-11 |
| Platelet (×103/μL) | 10 | 150-400 |
| Differential count | P91.2 L7.9 M0.2 | |
| APTT (seconds) | 30.3 | 28-38.4 |
| PT (seconds) | 10.5 | 10-14 |
| Fibrinogen (mg/dL) | 247 | 200-400 |
| INR | 0.87 | 0.96-1.23 therapeuticrange -2-3 |
| RFT (Urea/Creatinine) (mg/dL) | 22/0.6 | 19-43/0.66-1.25 |
| Bilirubin (Total/Direct) (mg/dL) | 0.4/0.2 | 0.3-1.2/0-0.2 |
| Total Protein/Albumin (mg/dL) | 6.6/3.5 | 6.3-8.2/3.5-5.2 |
| SGOT/SGPT (U/L) | 22/18 | 0-35/14-36 |
| ANA Profile | Negative | |
| APLA Profile | Negative | |
| HIV | Non reactive | |
| HBsAG | Non reactive | |
| HCV | Non reactive | |
| Blood group | A positive | |
| HbA1C (%) | 8.5 | 4-6.5 |
| DCT | Negative | |
| CT/BT (minutes) | 10/3 | 8-15/2-7 |
WBC: White blood cell; APTT: Activated partial thromboplastin time; PT: Prothrombin time; INR: International normalised ratio; RFT: Renal function test; SGOT: Serum glutamic-oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase; ANA: Antinuclear antibody; APLA: Antiphospholipid antibodies; HIV: Human immunodeficiency virus; HBsAg: Hepatitis B surface antigen; HCV: Hepatitis C virus; DCT: Direct coombs test; BT/CT: Bleeding time and clotting time
She was started on inj. Dexamethasone 40 mg i.v. OD for four days, and serial platelet count monitoring was done along with daily General Random Blood Sugar (GRBS) monitoring. At admission, the platelet count was 10×103/μL, 4 units of Random Donor Platelets (RDP) were transfused, and the post-transfusion platelet count was 15×103/μL. On the third day of admission, a dip in the platelet count was noted to be 12×103/μL [Table/Fig-1b]. She had an episode of epistaxis on the fourth day of admission and was managed conservatively with nasal packing and inj. tranexamic acid 1 g in 100 mL normal saline. She was later shifted to the critical care unit, where four units of RDP were transfused, and ENT consultation was sought. Cardiology consultation was also requested for foetal ECHO, which showed no significant structural heart disease. Antinuclear Antibody (ANA) profile immunoblot test and Antiphospholipid Antibody (APLA) profile were negative.
Shows the platelet count monitoring done on a daily basis from the day of admission till the day of discharge.
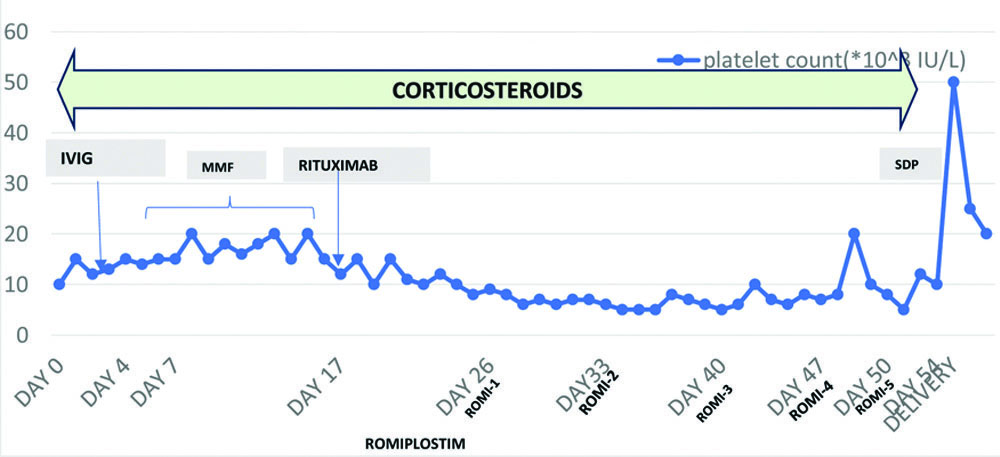
As part of ITP management, the patient was given one dose (50 g) of IVIG along with Tab Wysolone 40 mg OD for two weeks and tapered over the due course. There was an increment in the platelet count up to 20×103/μL. The patient’s condition improved, and she was shifted out of the ICU. She was then started on Tab Mycophenolate mofetil 500 mg BD, continued for five days, and the platelet count remained between 10-15×103/μL. Later, the patient was continued on oral steroids for the rest of the hospital course. The platelet count during the next 10 days remained within the range of 15-20×103/μL. In the meanwhile, as per the rheumatology consultation, Inj. rituximab 100 mg i.v. was started and continued for two weeks (as Rituximab is an anti-CD-20 monoclonal antibody and also an elective choice of treatment for refractory ITP). This caused a decline in platelet counts (5-8×103/μL). Hence, Inj. Romiplostim 250 μg/0.5 mL s/c was advised weekly once (Total 5 doses given).
At 35 weeks, serial Cardiotocography (CTG) was done, which showed high baseline values and poor variability. Consequently, induction of labour was planned. Neonatal Intensive Care Unit (NICU) counselling was provided regarding the risks of preterm delivery, bleeding, and the potential need for NICU admission. A surgical consultation was scheduled in anticipation of a possible need for splenectomy in case a Lower Section Caesarean Section (LSCS) had to be performed. However, splenectomy was postponed by the Haematology team due to the clinician’s decision based on the risk versus benefit analysis of the procedure.
The platelet counts fluctuated between 6000-8000/μL before induction, prompting the arrangement of Single Donor Platelets (SDP) from the transfusion medicine department due to the risk of bleeding. During labour induction, two units of SDP were transfused, followed by one unit each intrapartum and postpartum, maintaining platelet counts between 25-50×103/μL. A live female baby weighing 2.63 kg was delivered via normal vaginal delivery with an APGAR score of 7/10 at one minute. The baby was handed over to a Neonatologist. No excessive bleeding was observed during delivery.
The patient was managed with intravenous antibiotics (inj. Ceftriaxone 1 gm IV for 5 days), analgesics (Tab Meftal spas twice daily for 5 days), and other supportive medications (Tab Pantop 40 mg once daily for 5 days, Tab Tranexa 500 mg twice daily for 5 days, Tab Nicardia retard 10 mg once daily for 7 days).
The baby was admitted to NICU for respiratory distress, specifically Transient Tachypnoea of the Newborn, requiring Continuous Positive Airway Pressure (CPAP) for 12 hours and then being weaned off support. The newborn’s [Table/Fig-2a,b] blood tests showed a decrease in platelet count to 20,000/μL, prompting the administration of IVIG and platelet concentrate (2 units of RDP). The baby also developed jaundice (S.bilirubin-14.44 mg/dL) and was placed under phototherapy, which effectively reduced the serum bilirubin levels. The platelet count at discharge was 50×103/μL.
Investigation of the baby.
| Parameters | Results | Reference value |
|---|
| Haemoglobin (g/dL) | 17.5 | 12-14 |
| Total WBC count (×103/μL) | 24.72 | 4-11 |
| Platelet (×103/μL) | 30 | 150-400 |
| Differential count | P67.5 L25.7 M4.7 E1.6 | |
| Chloride (mEq/L) | 97 | 98-107 |
| Potassium (mEq/L) | 5.1 | 3.5-5.1 |
| Sodium (mEq/L) | 135 | 137-145 |
| NBIL | 15.2 | |
| T4 (μg/dL) | 12.13 | 5.93-13.09 |
| TSH (uIU/mL) | 4.59 | 0.79-5.85 |
| Peripheral blood smear (pathology) | Impression:- Normocytic normochromic blood picture with occasional normoblasts- WBC count normal with neutrophils and lymphocytes in equal proportion- Severe thrombocytopenia | |
| RFT (Urea/Creatinine) (mg/dL) | 30/1.2 | 19-43/0.66-1.25 |
| Bilirubin (Total/Direct) (mg/dL) | 14.4/0.0 | 0.3-1.2/0-0.2 |
| Total Protein/albumin (mg/dL) | 7.3/3.9 | 6.3-8.2/3.5-5.2 |
| SGOT/SGPT (U/L) | 42/25 | 0-35/14-36 |
| Blood group | A positive | |
| CRP-PAED | 0.05 | |
| Lactate (mg/dL) | 67.5 | 6.3-18.9 |
WBC: White blood cell; RFT: Renal function test; SGOT: Serum glutamic-oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase; NBIL: Neonate billirubin; TSH: Thyroid stimulating hormone; CRP: C-reactive protein
Platelet count trend in the baby.

For the mother, T. Prednisolone (Wysolone) was gradually reduced to 10 mg, and T. Azathioprine (Azoran) 25 mg was initiated upon discharge. The patient was advised to follow-up in the haematology Outpatient Department (OPD) after one month, but she did not attend the appointment. Two months later, she presented with vaginal bleeding at the gynaecology OPD and was prescribed Tab Novelon 1 tablet once daily for 20 days. Subsequently, the patient did not return for any further follow-ups.
Discussion
Thrombocytopenia can occur in up to 7-11% of pregnancies, and ITP is a typical reason for thrombocytopenia in the first and second trimesters of pregnancy, accounting for 1-4% of all cases of pregnancy-related thrombocytopenia [1]. ITP is a complex disease with heterogeneity and a lack of diagnostic indicators, making treatment decisions difficult. Perhaps the easiest part of management is how ITP presents. If the platelet count is very low and there are no other findings, steroids are a common treatment option worldwide [2].
In a pregnancy with ITP, as the pregnancy progresses, it is necessary to increase the platelet count to a safe level to support the method of delivery, assisting smooth regional anaesthesia and analgesia, and reducing the likelihood of perinatal complications. The International Consensus Report recommends starting treatment when the platelet count falls below 20-30×103/μL in the first and second trimesters, with an intervention planned or in symptomatic patients [3]. There is a lack of research on the use of second and third-line treatments for ITP during pregnancy, which is yet another problem [3]. In the treatment of ITP with multidrug combinations, drugs with different mechanisms of action may result in additive or even synergistic action. Medicines like IVIG, corticosteroids, and rituximab reduce immune-mediated destruction, while the TPO agonist (romiplostim) causes an increment in platelet production and might be utilised simultaneously [4]. Due to their efficacy and low cost, many consider corticosteroids to be the first-line treatment for ITP in pregnancy. The mechanisms of action of corticosteroids are the inhibition of phagocytosis of opsonised platelets and impairment of autoantibody production. The typical therapeutic dose of prednisone is 1 mg/kg/day (based on prepregnancy weight), which can be gradually reduced after achieving a response. Alternatively, IVIG can be the first-line therapy for pregnancy-associated ITP, especially when a long duration of therapy may not be required. Treatment with high-dose (i.e., 2 g/kg over 2-5 days) IVIG is an effective means of rapidly raising the platelet count. American Society of Haematology (ASH) guidelines consider IVIG to be an appropriate first-line agent for severe thrombocytopenia or thrombocytopenic bleeding in the third trimester [5].
Response to IVIG tends to be transient; however, multiple courses of therapy may be required, incurring significant costs and inconveniences for patients [6]. Azathioprine and cyclosporine A can also be used during pregnancy; however, they have a slow initiation of activity and low response rates. An elective treatment of choice may be Rituximab (anti-CD20 monoclonal antibody), as it has an overall response rate of 62.5% that can last from 2 to 48 months. It also has the potential to prevent the need for splenectomy in 40% of patients with ITP. In RhD-positive patients, anti-RhD immunoglobulin could have been used as well. However, despite successful outcomes, its use during pregnancy is not recommended due to rare life-threatening complications of intravascular haemolysis and acute renal failure [7].
Splenectomy is considered the second or third line of treatment during pregnancy, especially in rare refractory ITP cases. Roughly, 70-75% of pregnant patients with ITP respond to splenectomy, but in some cases, the platelet count shows only a minor increment, and the majority of relapses are observed in the first half-year postsplenectomy. In cases refractory to splenectomy, Romiplostim, a thrombopoietin receptor agonist, can be administered. It stimulates platelet production by activating megakaryocytes in the bone marrow. The mean therapeutic dose is 3 to 4 μg/kg per week, with a reaction time of around five to eight days and reaction rates at 95%. The main foetal complications are thrombocytosis, reversible bone marrow fibrosis, and thromboembolic events [7].
Severe thrombocytopenia in babies born to mothers with ITP is very rare. IVIG and platelet transfusions are recommended for those with platelet counts less than 30×103/μL. Mortality is seen in less than 1% of cases, and intracranial haemorrhages in less than 1.5% of cases [8].
Therefore, in ITP during pregnancy with no bleeding manifestations, it is crucial to closely monitor the platelet count monthly during the initial two trimesters, fortnightly during the third trimester, and weekly when nearing term. Treatment should be initiated when the platelet count is less than 20-30×103/μL before 36 weeks, or in the presence of bleeding events during any trimester, or when the platelet count is less than 30-50×103/μL at 36 weeks [9].
Accurate diagnosis of thrombocytopenia during pregnancy is important for effective treatment strategies, and a multidisciplinary team of haematologists and intensivists is critical for prenatal care and delivery [10]. Similarly, a tool that can accurately estimate an infant’s platelet count at birth would be very useful. Unfortunately, methods such as scalp sampling or percutaneous cord blood collection are no longer used for this purpose. Platelet counts in collected samples do not necessarily correlate with platelet counts subsequently measured in the newborn, and these procedures are associated with an unacceptable risk of bleeding and/or preterm birth [11].
Conclusion(s)
The management of refractory ITP during pregnancy is exceptionally challenging and requires a multidisciplinary approach and individualised treatment plans. This case demonstrates the importance of continuous monitoring, adaptive treatment strategies, and the use of advanced therapies such as rituximab and romiplostim. Despite significant fluctuations in platelet counts and episodes of bleeding, a successful maternal and neonatal outcome was achieved. This underscores the potential benefits of emerging therapies and the necessity for tailored protocols in managing complex cases of ITP during pregnancy. Ongoing research and clinical collaboration are essential for optimising outcomes in such high-risk scenarios.
WBC: White blood cell; APTT: Activated partial thromboplastin time; PT: Prothrombin time; INR: International normalised ratio; RFT: Renal function test; SGOT: Serum glutamic-oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase; ANA: Antinuclear antibody; APLA: Antiphospholipid antibodies; HIV: Human immunodeficiency virus; HBsAg: Hepatitis B surface antigen; HCV: Hepatitis C virus; DCT: Direct coombs test; BT/CT: Bleeding time and clotting time
WBC: White blood cell; RFT: Renal function test; SGOT: Serum glutamic-oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase; NBIL: Neonate billirubin; TSH: Thyroid stimulating hormone; CRP: C-reactive protein
[1]. Agarwal N, Mangla A, Thrombopoietin receptor agonist for treatment of immune thrombocytopenia in pregnancy: A narrative reviewTher Adv Hematol 2021 12:01-10.10.1177/2040620721100113933796239 [Google Scholar] [CrossRef] [PubMed]
[2]. Bussel JB, Garcia CA, Diagnosis of immune thrombocytopenia, including secondary forms, and selection of second-line treatmentHaematologica 2022 107(9):2018-36.10.3324/haematol.2021.27951335708136 [Google Scholar] [CrossRef] [PubMed]
[3]. Harrington P, Nelson-Piercy C, Williamson C, Cooper N, Kesse-Adu R, Robinson S, Refractory severe immune thrombocytopenia in a twin pregnancyObstet Med 2018 11(1):35-38.10.1177/1753495X1770918829636813 [Google Scholar] [CrossRef] [PubMed]
[4]. Chon AH, Chan R, Lee RH, Kwong K, Wertheimer FB, Weitz IC, Multidrug therapy for refractory immune thrombocytopenia in pregnancyObstet Gynecol 2020 135(3):723-27.10.1097/AOG.000000000000369932028499 [Google Scholar] [CrossRef] [PubMed]
[5]. Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Crowther MA, The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopeniaBlood 2011 117(16):4190-207.Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2784425/10.1182/blood-2010-08-30298421325604 [Google Scholar] [CrossRef] [PubMed]
[6]. Stavrou E, McCrae KR, Immune thrombocytopenia in pregnancyHematol Oncol Clin North Am 2009 23(6):1299-316.PMCID: PMC278442510.1016/j.hoc.2009.08.00519932435 [Google Scholar] [CrossRef] [PubMed]
[7]. Decroocq J, Marcellin L, Le Ray C, Willems L, Rescue therapy with romiplostim for refractory primary immune thrombocytopenia during pregnancyObstet Gynecol 2014 124(2 Pt 2 Suppl 1):481-483.10.1097/AOG.000000000000037125004319 [Google Scholar] [CrossRef] [PubMed]
[8]. Gilmore K, McLintock C, Maternal and fetal outcomes of primary immune thrombocytopenia during pregnancy: A retrospective studyObstet Med 2017 11(1):12-16.10.1177/1753495x1772740829636808 [Google Scholar] [CrossRef] [PubMed]
[9]. Nicolescu A, Vladareanu AM, Voican I, Onisai M, Vladareanu R, Therapeutic options for Immune Thrombocytopenia (ITP) during pregnancyMaedica (Bucur) 2013 8(2):182-88. [Google Scholar]
[10]. Naumchik A, Davydova I, Lymanska A, Oliynyk V, Clinical case of severe immune thrombocytopenia during pregnancyUkr J Perinatol Pediatr 2023 94:142-44.10.15574/pp.2023.94.142 [Google Scholar] [CrossRef]
[11]. González-Porras J, Palomino D, Vaquero-Roncero L, Bastida J, Bleeding complications associated with pregnancy with primary immune thrombocytopenia: A meta-analysisTH Open 2022 6(3):e230-e237.10.1055/a-1837-758136046200 [Google Scholar] [CrossRef] [PubMed]