Surgical interventions carried out to reduce human suffering result in inevitable consequences such as pain and distress to the patient. Controlling acute pain that follows tissue injury after surgery is important during the postoperative period as well as in preventing chronic postsurgical pain, which can develop in almost 10% of patients [1]. Ineffective pain management can result in negative clinical and psychological outcomes such as restlessness causing hypoxemia, coronary ischaemia, myocardial infarction, poor wound healing, insomnia, decreased quality of life, and demoralisation, which further increases morbidity and mortality [2]. Thyroidectomy is one of the most frequent surgical treatments performed for various thyroid pathologies that cause mild to moderate pain during the first 24 hours postsurgery, hence requiring adequate postoperative pain relief to augment patient recovery and satisfaction [3,4]. Thyroid surgeries, generally carried out under general anaesthesia, require relatively deeper anaesthesia due to the combined effects of surgery and frequent stimuli to the trachea due to movements of the endotracheal tube throughout surgery and can lead to complications such as swallowing difficulty, sore throat, nausea, and vomiting along with pain [5]. Postoperative pain management is usually by either administration of nonsteroidal anti-inflammatory drugs, which may be ineffective in pain relief and increase the risk of bleeding postoperatively, or opioids, which have adverse effects like nausea, vomiting, sedation, and inadequate respiration, worsening the clinical condition of the patient [6].
Loco-regional methods of anaesthesia can alleviate postoperative pain and prevent sensitisation of the central and peripheral nervous system due to a longer duration of action, thus preventing the development of chronic pain without the side effects of systemic analgesics [7-9]. A recently introduced highly selective alpha-2 agonist, dexmedetomidine, has been evaluated as an adjunct in peripheral nerve blocks and is reported to be safe and effective in prolonging the action of the peripheral blocks [10,11]. Glucocorticoids have a prerequisite to bind to ligands within the cell and be transported into the nucleus, where they have an effect on Deoxyribonucleic Acid (DNA) transcription causing anti-inflammatory action. Dexamethasone is proven to amplify the action of local anaesthetics by modifying the function of potassium channels in the excitable cells, which halts the transmission along with causing local vasoconstriction, hence prolonging the duration of nerve blocks [12].
Literature on the comparison of the effect of Dexamethasone and Dexmedetomidine as an Adjuvant to Bupivacaine for BSCPB is limited. Hence, the present study aimed at evaluating the influence of adjuvants on the block and comparing the effects of adjuvants on intraoperative haemodynamic stability, postoperative VAS scores, duration of the block, and postoperative analgesic consumption.
The primary objective of present study was to assess postoperative VAS scores in both groups and estimate the duration of the block. The secondary objective was to monitor the haemodynamic stability intraoperatively, study the cumulative dose of rescue analgesia utilised in the postoperative hours, and observe any complications.
Materials and Methods
The present randomised, double-blinded clinical trial was conducted in the Department of Anesthesiolgy at teritary care centre, BLDE (Deemed to be University) Shri BM Patil Medical College, Hospital and Research Centre in Vijayapur, Karnataka, India for a duration of one year, from April 2023 to March 2024, after approval from the Institutional Ethical Committee (BLDE (DU)/IEC/ 790/ 2022-23). The study is registered with Clinical Trail of Registry India (CTRI) (CTRI/2023/03/050683).
Inclusion criteria: Patients between the age of 18 to 60 years, classified as ASA grades I-II, and scheduled for elective thyroid surgeries under general anaesthesis were included in the study.
Exclusion criteria: Patient refusal, impaired thyroid function test, local site infection, allergy to drugs used in present study, coagulopathies, patients with heart block, or patients on adrenoreceptor agonist or antagonist treatment were excluded from the study.
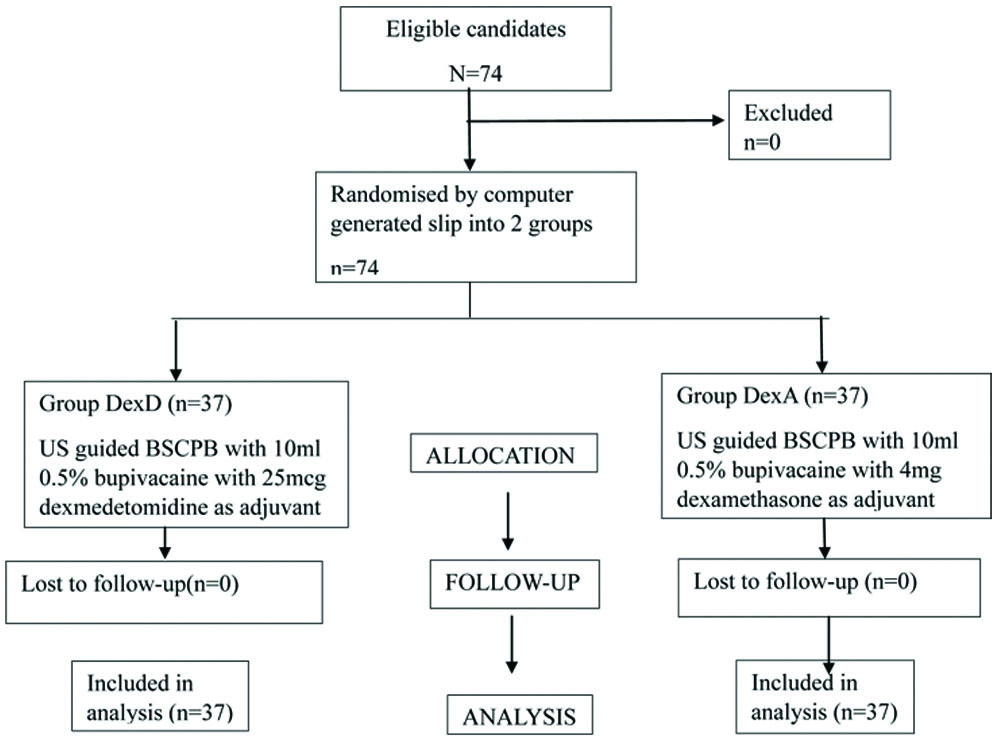
Sample size calculation: A total 74 patients scheduled for elective thyroid surgery were randomised by computer-generated slips into two groups named DexD and DexA. The sample size was calculated using G* Power version 3.1.9.4 software as 74 to achieve a power of 99% for detecting a difference in means with a 5% level of significance.
Study Procedure
Patients were sensitised about the study method, procedures, and educated about the VAS during the preanaesthetic exam. Written informed consent was obtained. Patients were randomised using a computer-generated slip. After confirming nil per oral status, patients were shifted to the operating theatre. Patients were monitored for Systolic and Diastolic Blood Pressure (SBP, DBP), along with Mean Arterial Pressure (MAP), oxygen saturation, and HR. Baseline vitals were noted. Patients received premedication including midazolam (0.02 mg/kg), ondansetron (0.15 mg/kg), and glycopyrrolate (0.02 mg/kg). After preoxygenation, general anaesthesia was induced with fentanyl (2 μg/kg), propofol (2 mg/kg), and atracurium (0.5 mg/kg). Patients were intubated and ventilated. General anaesthesia was maintained with isoflurane, oxygen, nitrous oxide, and atracurium as per requirement. Bilateral Superficial Cervical Plexus Block (BSCPB) was administered under ultrasound guidance to all patients after proper positioning, before the surgical incision. The DexD group received an ultrasound-guided block of 10 mL of 0.5% bupivacaine with 25 μg of dexmedetomidine as the adjuvant on both sides, totaling 50 mcg [13]. The DexA group received 10 mL of 0.5% bupivacaine with 4 mg of dexamethasone on both sides, totaling 8 mg [12]. The drug was prepared by the anaesthesia technician according to computer-generated allotment, and the block was performed by the anaesthesiologist. The patient and performing anaesthesiologist were blinded to the drug administered. Intraoperatively, HR, SBP, DBP, and MAP were monitored every 10 minutes after 30 minutes from the time of the block upto 120 minutes. At the completion of the procedure, muscle relaxation was reversed with neostigmine and glycopyrrolate, and patients were extubated. VAS scores were assessed for both groups at 30 minutes, 1, 2, 6, 8, 12, and 24 hours postoperatively. Diclofenac 75 mg [14] in 100 mL normal saline intravenous infusion was used as rescue analgesia when the VAS scores were more than 4. The time period before the first rescue analgesia request was documented along with the total analgesic dose consumed postoperatively within 24 hours for both groups. Any complications such as nausea, vomiting, hoarseness of voice, and throat discomfort were recorded [Table/Fig-1].
Consodilated Standards of Reporting Trails (CONSORT) flow diagram.
Statistical Analysis
The data was entered into a Microsoft Excel sheet, and statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS version 20.0). The results were presented as Mean±Standard Deviation (SD), percentages, and bar graphs. An independent sample t-test was utilised for normally distributed continuous variables. The Mann-Whitney’s U test was used for variables that were not normally distributed. The Chi-square test/Fisher’s-Exact test was applied to compare categorical variables. Statistical significance was considered if the p-value was <0.05.
Results
The age of participants in both groups was comparable. The mean age in Group DexD was 42.59±8.64 years, and in Group DexA it was 45.40±8.96 years with a p-value of 0.219. Most of the patients were between 31-60 years of age. The female population was higher 65 (87.9%) than the male population 9 (12.1%) in present study. The comparison of the male to female ratio between the groups was assessed using a Chi-square test with a p-value of 0.649, which was not significant [Table/Fig-2].
The demographic profile of two groups.
| Age group(in years) | DexD | DexA |
|---|
| Male | Female | Total | % | Male | Female | Total | % |
|---|
| 18-20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21-30 | 0 | 2 | 2 | 5.4 | 0 | 3 | 3 | 8.1 |
| 31-40 | 2 | 13 | 15 | 40.5 | 2 | 10 | 12 | 32.4 |
| 41-50 | 1 | 12 | 13 | 35.1 | 1 | 9 | 10 | 27 |
| 50-51 | 3 | 4 | 7 | 18.9 | 0 | 12 | 12 | 32.4 |
| 51-60 | 6 | 31 | 37 | 100 | 3 | 34 | 37 | 100 |
DexD: Dexmedetomidine group; DexA: Dexamethasone group; Age: in years; n: Number of patients; %: Percentage
Mean VAS scores between the two groups showed a statistically significant difference. Group DexD had significantly lower VAS scores at 30 minutes, 1, 2, 6, and 8 hours with a p-value <0.05 [Table/Fig-3].
Comparison of mean VAS scores at predefined time intervals among two groups.
| Time interval | Group DexD (mean±SD) | Group DexA (mean±SD) | p-value |
|---|
| 30 min | 0.62±0.53 | 0.91±0.42 | 0.011* |
| 1 hour | 0.83±0.49 | 1.05±0.22 | 0.015* |
| 2 hours | 1.18±0.39 | 1.45±0.64 | 0.031* |
| 6 hours | 1.37±0.58 | 1.94±0.65 | 0.0002* |
| 8 hours | 2.108±0.68 | 2.72±0.68 | 0.0002* |
| 12 hours | 3.02±0.78 | 2.73±0.64 | 0.259 |
| 24 hours | 1.13±0.34 | 1.24±0.48 | 0.342 |
Min: Minutes; SD: Standard; VAS scores are expressed in terms of mean±SD; Student’s t-test is used to compare the data; *Statistically significant
When the mean time period before the first rescue analgesic dose request (in minutes) between groups was compared, the data was statistically valid with a p-value of 0.001. In Group DexD, the mean time taken in minutes before the first rescue analgesic dose was 688.37±55.75, and in Group DexA it was 593.64±72.56. Group DexD had significantly longer postoperative analgesia when compared to Group DexA [Table/Fig-4].
Comparison of mean time period before the first rescue analgesic dose among the two groups.
| Time taken before first rescue analgesic dose (in mins) | Mean±SD | p-value |
|---|
| Group DexD | 688.37±55.75 | 0.001* |
| Group DexA | 593.64±72.56 |
Min: minutes; SD: Standard deviation; the data is compared using student’s t-test and is expressed as mean±SD; *Statistically significant
Intraoperative SBP, DBP, MAP, and HR were measured every 10 minutes starting from 30 minutes from the block time till the completion of the procedure or up to 120 minutes, whichever was the earliest. The mean SBPs had a statistically notable difference between the two groups. Group DexD showed significantly lower mean SBPs at all intervals (p-value <0.05). When mean DBPs were compared, Group DexD had significantly lower mean DBPs at 30, 40, 50, 60, 70, and 80 minutes than Group DexA with a p-value <0.05 [Table/Fig-5]. Similarly, the mean MAP was suggestively lesser in the DexD group when compared with that of the DexA group at each time interval with a p-value less than 0.05. The mean HR at all time intervals was substantially lesser in Group DexD compared to Group DexA with a p-value <0.05 [Table/Fig-6].
Comparing mean intraoperative SBP and DBP among two groups at predefined time intervals.
| Time intervals | DexD SBP (mean±SD) | DexA SBP (mean±SD) | p-value | DexD DBP (mean±SD) | DexA DBP (mean±SD) | p-value |
|---|
| 30 min | 105.81±8.49 | 115.51±11.57 | <0.001* | 68.42±6.85 | 74.59±8.94 | 0.001* |
| 40 min | 104.54±8.97 | 115.92±1028 | <0.001* | 69.14±7.96 | 75.73±6.74 | <0.001* |
| 50 min | 104.16±8.25 | 116.16±10.78 | <0.001* | 69.16±7.03 | 75.24±6.81 | <0.001* |
| 60 min | 105.86±9.36 | 116.76±10.17 | <0.001* | 69.65±8.01 | 74.78±7.03 | 0.005* |
| 70 min | 107.14±9.34 | 118.54±8.92 | <0.001* | 70.43±6.92 | 73.11±7.07 | 0.01* |
| 80 min | 108.14±10.97 | 118.14±8.61 | <0.001* | 70.41±7.50 | 74.94±6.26 | 0.007* |
| 90 min | 111.33±9.04 | 117.24±9.16 | 0.009* | 71.36±6.11 | 73.18±5.88 | 0.21 |
| 100 min | 111.63±6.30 | 118.10±10.29 | 0.004* | 72.69±5.69 | 74.23±6.74 | 0.33 |
| 110 min | 113.24±8.57 | 119.28±11.31 | 0.003* | 72.45±6.97 | 74.88±8.14 | 0.24 |
| 120 min | 113.28±8.55 | 120.08±11.51 | 0.002* | 73.38±6.19 | 75.21±8.51 | 0.37 |
Min: minutes; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; SD: Standard deviation
Data are expressed in terms of mean±SD. Student’s t-test is used to compare the data
*Statistically significant
Comparison of intraoperative MAP and HR among two groups at predefined time intervals.
| Time interval | DexD MAP (Mean±SD) | DexA MAP (Mean±SD) | p-value | DexD HR (Mean±SD) | DexA HR (Mean±SD) | p-value |
|---|
| 30 min | 80.27±7.46 | 89.43±10.63 | <0.001* | 74.51±10.76 | 81.38±10.75 | 0.008* |
| 40 min | 79.65±8.37 | 89.08±7.80 | <0.001* | 72.89±11.16 | 82.03±12.44 | 0.001* |
| 50 min | 79.51±7.72 | 89.38±9.18 | <0.001* | 72.14±10.71 | 81.51±12.53 | 0.001* |
| 60 min | 80.03±8.22 | 90.05±9.85 | <0.001* | 72.27±11.04 | 82.41±12.25 | <0.001* |
| 70 min | 81.32±9.04 | 90.43±9.67 | <0.001* | 71.11±10.73 | 81.3±12.10 | <0.001* |
| 80 min | 81.84±10.51 | 91.53±9.37 | <0.001* | 72.68±11.16 | 81.61±11.45 | 0.001* |
| 90 min | 84.89±9.23 | 90.52±9.20 | 0.014* | 73.66±9.23 | 80.58±10.88 | 0.003* |
| 100 min | 84.59±6.02 | 90±9.66 | 0.01* | 74.19±9.58 | 81±9.29 | 0.006* |
| 110 min | 87.98±9.91 | 93.56±9.76 | 0.025* | 76.48±9.66 | 81.04±9.42 | 0.008* |
| 120 min | 86.14±8.50 | 93.25±9.88 | 0.007* | 75.48±8.60 | 80.58±9.18 | 0.04* |
Min: minutes; MAP: Mean arterial pressure; HR: Heart rate; SD: Standard deviation
Data are expressed in terms of mean±SD. Student’s t-test is used to compare the data
*Statistically significant
The cumulative dose of analgesic (diclofenac in mg) consumed in 24 hours in Group DexD was calculated to be 81.08±20.754 mg. The cumulative dose of analgesic (diclofenac in mg) required in 24 hours in Group DexA was calculated to be 104.17±44.921 mg. Group DexD required significantly less dose of analgesic with a p-value of 0.006 [Table/Fig-7].
Cumulative postoperative analgesia utilised (in 24 hours).
| Cumulative dose of analgesia utilised in 24 hours postoperatively | Mean±SD(in mg) | p-value |
|---|
| Group DexD | 81.08±20.754 | 0.006* |
| Group DexA | 104.17±44.921 |
mg: Milligrams; SD: Standard deviation
Data are expressed in terms of mean±SD. Student’s t-test is used to compare the data
*Statistically significant
Total 20 (54.05%) patients in Group DexD reported nausea while only 3 (8.1%) patients in Group DexA complained of nausea postoperatively. Group DexA was observed to have a significantly reduced incidence of nausea postoperatively with a p-value of 0.002 [Table/Fig-8].
Comparison of incidence of nausea and vomiting among two groups.
| Complications | Group DexD | Group DexA | p-value |
|---|
| Yes | No | Yes | No |
|---|
| n | % | n | % | n | % | n | % |
|---|
| Nausea | 20 | 54.05 | 17 | 45.95 | 3 | 8.1 | 34 | 91.9 | 0.002* |
| Vomiting | 16 | 43.24 | 21 | 56.76 | 0 | 0 | 37 | 100 | 0.001* |
%: Percentage; n: Number of patients
Data are expressed in terms of percentage and Chi-square test is used to compare the same; *Statistically significant
16 (43.24%) patients in Group DexD had complaints of vomiting, while none of the patients in Group DexA reported any episodes of vomiting postoperatively. Group DexA was observed to have a significantly reduced incidence of vomiting postoperatively, with a p-value of 0.001 [Table/Fig-8].
In Group DexD, 6 (16.20%) patients reported having throat discomfort, while 31 (83.80%) did not report any. In Group DexA, 10 (27%) patients experienced throat discomfort, and 27 (73%) did not experience such symptoms. The occurrence of throat discomfort was similar between the two groups (p-value=0.259).
In Group DexD, 1 (2.7%) patient complained of hoarseness of voice, while 36 (97.30%) did not have such complications. In Group DexA, none of the patients complained of hoarseness of voice. The incidence of hoarseness of voice was comparable between the groups (p-value=0.314).
Discussion
The present trial demonstrated that dexmedetomidine effectively prolonged the duration of action of BSCPB (p-value=0.001) when used as an adjuvant to Bupivacaine, with significantly better haemodynamic stability, lower postoperative VAS scores up to eight hours (p-value=0.0002), and less postoperative analgesic consumption (p-value=0.006). However, postoperative nausea (p-value=0.002) and vomiting (p-value=0.001) incidence were significantly reduced by the addition of dexamethasone to bupivacaine.
In present study, intraoperative haemodynamic variables such as SBP, MAP, and HR were significantly lower in Group DexD than in Group DexA at all intervals, along with significantly lower DBP in Group DexD at 30, 40, 50, 60, 70, and 80-minute intervals. Jain N et al., and Hassan AH et al., in their studies, described the same trends in the haemodynamic parameters being lower in the dexmedetomidine group compared to the dexamethasone group [15,16].
The mean VAS scores between the two groups were noted to be significantly lower in Group DexD than in Group DexA at intervals of 30 minutes, 1, 2, 6, and 8 hours, whereas they were comparable at 12 and 24 hours. The same is reflected in a trial conducted by Thakur J et al., comparing dexmedetomidine and dexamethasone as adjuvants to bupivacaine for TAP block in 120 patients and observed lower VAS scores in patients who received dexmedetomidine as an adjuvant [17]. A different observation was made in two different trials conducted by Jain N et al., and Gao Z et al., who observed comparable VAS scores when they assessed dexmedetomidine and dexamethasone as adjuvants with bupivacaine for BSCPB and Erector Spinae Plane Block (ESPB), respectively [15,18].
The observations regarding the analgesic efficacies of the mentioned adjuvants exhibit inconsistency in results when the existing literature is reviewed. In the present study, the mean time period between the administration of BSCPB and the first rescue analgesic dose request was observed to be prolonged in Group DexD (688±55.75 min) compared to Group DexA (593.64±72.56 min) with statistical significance (p-value=0.01). Hence, dexmedetomidine prolonged the time period before the first rescue analgesic dose, providing a longer period of analgesia than dexamethasone when added as an ancillary to local anaesthetics for BSCPB. Similar observations were made by Mohammed Ali DS et al., in their study conducted on 84 female patients undergoing Total Abdominal Hysterectomy (TAH) [19]. The study concluded that dexmedetomidine, when compared to dexamethasone as an adjunct to bupivacaine for ESPB, prolonged the duration of analgesia. In line with the observations made in this trial, Singla N et al., observed that adding dexmedetomidine to bupivacaine for TAP block resulted in a prolonged duration of analgesia [20]. However, research conducted by Adinarayanan S et al., had a contrasting conclusion. In this study, it was observed that dexamethasone, when added to bupivacaine, proved to be superior to dexmedetomidine and lengthened the duration of the brachial plexus block (supraclavicular approach) [21]. Similarly, research by Elbahrawy K and EL- Deeb A determined that when ropivacaine 0.2% was supplemented with dexamethasone for BSCPB, it resulted in an extended duration of the block and decreased the systemic analgesia requirement [22].
The mean total postoperative analgesic dose with diclofenac consumed in the first 24 hours was significantly lower in Group DexD (81.08±20.75 mg) than in Group DexA (104.17±44.92 mg) with a p-value of 0.006. Thakur J et al., in their trial made identical observations [17]. The number of rescue analgesic doses requested was significantly lower in patients who were administered dexmedetomidine at 1 mcg/kg with bupivacaine for TAP block than in those who received dexamethasone at 0.1 mg/kg. Hassan AH et al., Mohammed Ali DS et al., also had similar outcomes in their respective trials [16,19]. In line with the study by Jain N et al., [15], it was observed that when dexamethasone was the adjuvant to bupivacaine in patients belonging to Group DexA, there was a significant alleviation in the incidence of nausea (p-value=0.002) and vomiting (p-value=0.001) postoperatively when compared to Group DexD, who received dexmedetomidine. Incidences of throat discomfort and hoarseness of voice were statistically insignificant between both groups (p-value >0.05).
The strength of the present study was that the BSCPB was performed under Ultrasonography (USG) guidance, which ensured precision and accuracy with fewer chances of complications. The duration of the block was increased due to the addition of the adjuvants. Intraoperative haemodynamic stability was well maintained throughout the surgery.
Limitation(s)
The present study is single-centred, with a limited sample size. Secondly, present trial only studied specific doses of drugs-0.5% bupivacaine, 25 mcg dexmedetomidine, and 4 mg dexamethasone. The effects of the drugs and the block need further research in patients of ASA III and IV undergoing thyroid surgery. The impact of BSCPB on intraoperative anaesthetic consumption, effects of different doses of adjuvants on block characteristics, and other adverse effects of the block are potential areas for exploration in future studies. An extensive trial involving multiple centers along with larger sample sizes is required to increase the generalisability of the results to a broader population.
Conclusion(s)
Dexmedetomidine, as an adjuvant to Bupivacaine for BSCPB, considerably prolonged the duration of the block with lower VAS scores, improved intraoperative haemodynamic stability, and reduced postoperative analgesic consumption compared to dexamethasone. However, dexamethasone, as an adjuvant, significantly reduced the incidence of postoperative nausea and vomiting. Overall, BSCPB is an effective locoregional technique that can be used as one of the postoperative analgesic modalities for patients undergoing thyroid surgeries. When used with adjuvants, it has a prolonged duration of action.
DexD: Dexmedetomidine group; DexA: Dexamethasone group; Age: in years; n: Number of patients; %: Percentage
Min: Minutes; SD: Standard; VAS scores are expressed in terms of mean±SD; Student’s t-test is used to compare the data; *Statistically significant
Min: minutes; SD: Standard deviation; the data is compared using student’s t-test and is expressed as mean±SD; *Statistically significant
Min: minutes; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; SD: Standard deviation
Data are expressed in terms of mean±SD. Student’s t-test is used to compare the data
*Statistically significant
Min: minutes; MAP: Mean arterial pressure; HR: Heart rate; SD: Standard deviation
Data are expressed in terms of mean±SD. Student’s t-test is used to compare the data
*Statistically significant
mg: Milligrams; SD: Standard deviation
Data are expressed in terms of mean±SD. Student’s t-test is used to compare the data
*Statistically significant
%: Percentage; n: Number of patients
Data are expressed in terms of percentage and Chi-square test is used to compare the same; *Statistically significant