Incidence of Tarsal Tunnel Syndrome in Type II Diabetic Patients using Clinical, Radiological and Electrodiagnostic Studies in a Tertiary Care Hospital: A Cross-sectional study
Vipul Kumar1, Mohammed Alam Parwaz2, Surjeet Dwivedi3, Shrikant Manwatkar4, Shreyansh Chowdhry5, Rahul Merkhed6, Arvind Kumar7, Pallavi Dhanvijay8
1 Junior Resident, Department of General Surgery, Army Hospital (Research and Referral), New Delhi, India.
2 Professor, Department of Plastic Surgery, Army Hospital (Research and Referral), New Delhi, India.
3 Classified Specialist Surgery and Oncosurgery, Department of Oncosurgery, Command Hospital Air Force, Bengaluru, Karnataka, India.
4 Classified Specialist Surgery, Trauma Surgery and Critical Care, Department of General Surgery, Command Hospital Air Force, Bengaluru, Karnataka, India.
5 Classified Specialist Surgery, Department of General Surgery, Command Hospital Air Force, Bengaluru, Karnataka, India.
6 Classified Specialist Surgery and Vascular Surgery, Department of Vascular Surgery, Command Hospital Air Force, Bengaluru, Karnataka, India.
7 Classified Specialist Surgery and Neurosurgery, Department of Neurosurgery, Command Hospital Air Force, Bengaluru, Karnataka, India.
8 Graded Specialist Pharmacology, Department of Pharmacology, Medical Training Centre, Bengaluru, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shrikant Manwatkar, Department of General Surgery, Command Hospital Air Force, Cambridge Road, Bengaluru-560007, Karnataka, India.
E-mail: shrikantxy@gmail.com
Introduction
Diabetic foot is one of the most devastating complications of diabetes and is the leading cause of lower limb amputations. Patients with a long-standing history of diabetes mellitus often experience symptoms of pain, burning sensation, numbness, and paraesthesia in the heel and feet. These symptoms may be due to compression of the medial plantar nerve, a branch of the tibial nerve, within the tarsal tunnel.
Aim
To evaluate the incidence of Tarsal Tunnel Syndrome (TTS) in patients suffering from Type 2 Diabetes Mellitus (T2DM).
Materials and Methods
This study was a cross-sectional analysis conducted at the Army Hospital (Research and Referral) in New Delhi, India, from October 2019 to April 2021. A total of 30 consecutive diabetic patients presenting with pain, burning, numbness, and paraesthesia in the heel or feet, with or without ulcers, were included. All patients were clinically evaluated using three-point sensory testing, Tinel’s sign at the tarsal tunnel, and assessment for the presence of ulcers on the foot. All patients underwent Nerve Conduction Velocity (NCV) studies. Imaging studies in the form of Magnetic Resonance Imaging (MRI) were performed for a complete work-up of these patients and further diagnosis of TTS. The incidence of TTS was evaluated using clinical, radiological and electrodiagnostic studies.
Results
Of the 30 patients enrolled, 20 were males (66.7%) and 10 were females (33.3%). The incidence of TTS among diabetic patients was found to be 14 (46.7%) based on electrodiagnostic criteria, 22 (73.3%) based on clinical evaluation, and 25 (83.3%) based on radiological findings. The mean HbA1c level was 8.5±1.04%. The most common imaging finding was oedema, observed in 83.3% of patients. Inflammation and ganglion cysts were seen in 13.3% and 10% of patients, respectively.
Conclusion
TTS is difficult to diagnose. MRI is a useful imaging modality to support the diagnosis. Electrodiagnostic studies can help confirm the diagnosis.
Diabetic neuropathy, Electrodiagnostic study, Entrapment neuropathy, Magnetic resonance imaging
Introduction
Diabetes is responsible for significant morbidity worldwide. The prevalence of diabetes was 171 million in 2000 and is projected to reach 366 million by 2030 globally. In India, more than 62 million individuals are affected by diabetes [1]. Diabetic foot is one of the most devastating complications of diabetes and is the leading cause of lower limb amputations. It poses a risk of disability, with frequent hospital stays often resulting in limb amputation. The prevalence of diabetic ulcers among diabetics worldwide is 4-10% [1]. The severity of diabetic foot can be assessed using the Wagner classification. In grade 0, there is no open ulcer. In grade 1, there is destruction of the thickness of the skin. Grade 2 ulcers penetrate through the skin, fat, and ligaments but do not affect bone. In grade 3, there is involvement of deeper tissues with abscesses, osteomyelitis, or tendonitis. Grade 4 consists of limited necrosis in the toes or the forefoot, while grade 5 indicates necrosis of the whole foot [2].
Diabetic neuropathy is a common accompanying factor in almost 90% of diabetic ulcers [2]. About 80% of these ulcers are neuropathic in origin, while the remaining 20% are neuroischaemic. According to the American Diabetes Association (ADA), a foot that has lost its protective sensation is considered to be at “risk” for ulceration [3]. Patients with a long-standing history of diabetes mellitus often experience symptoms such as pain, burning sensations, numbness, and paraesthesia in the heel and feet. These symptoms can result from compression of the medial plantar nerve, a branch of the tibial nerve, within the tarsal tunnel.
TTS is an entrapment neuropathy associated with the compression of structures within the tarsal tunnel [4]. It is similar to carpal tunnel syndrome of the wrist, although much less common [5]. TTS is associated with 80% of diabetic patients at risk for foot complications and often remains underdiagnosed, leading to a range of symptoms affecting the plantar margins of the foot [6]. Clinically, TTS can be identified by Tinel’s percussion test over the nerve, and hypoesthesia in the foot is assessed using the Semmes-Weinstein 10 g filament [7].
TTS is suspected based on the patient’s symptoms and diagnosed through electrodiagnostic tests. The following tests are required to assess TTS electrodiagnostically:
Distal Motor Latency (DML)
Sensory Conduction Velocity (SCV)
Amplitude of Sensory Nerve Action Potential (SNAP)
Radiological investigations are also important for identifying the causes of TTS, such as ganglion cysts and oedema of the surrounding structures. MRI is the most effective tool for assessing the contents of the tarsal tunnel, the flexor retinaculum, and the branches of the tibial nerve [8].
After reviewing the literature on various studies, authors found that TTS presents in a significant number of diabetic patients who exhibit subjective neuropathic symptoms in their feet, as many of the symptoms of TTS mimic those of diabetic neuropathy [9]. Therefore, TTS should be considered during the diagnostic work-up and management of diabetic patients. Consequently, this study was conducted to determine the incidence of TTS in patients suffering from T2DM. Hence, the aim of the study was to evaluate the effectiveness of different diagnostic modalities, including electrodiagnostic and radiological studies.
Materials and Methods
This study was a cross-sectional study conducted at the Army Hospital (Research and Referral) in New Delhi, India during the period from October 2019 to April 2021. Ethical clearance was obtained from the hospital’s ethical clearance committee (IEC No: 74/2019). Patients of any ethnicity or age from across the country who were referred to or brought to this hospital were enrolled. The patients came from both rural and urban backgrounds. Those who agreed to participate in the study provided signed written informed consent and satisfied the following inclusion criteria.
Inclusion criteria: Patients with a confirmed diagnosis of diabetes, with or without ulcers (early stages of Wagner classification [2] in the foot), at the Department of General Surgery of a tertiary care hospital were included in the study.
Exclusion criteria: Patients with diabetic foot selected for amputation and further flap coverage were excluded from the study, as these individuals would not be able to undergo testing for TTS after amputation. Patients with vascular insufficiency (Ankle-Brachial Pressure Index [ABPI] <0.7) were also excluded, as ischaemic pain due to vascular insufficiency would hinder the diagnosis of TTS. Patients in severe sepsis or those who were unstable were excluded from the study, as they would not be able to undergo testing for TTS. Additionally, patients with later stages of Wagner ulcers, as those would typically require amputation were excluded from the study.
Study Procedure
Thirty consecutive diabetic patients presenting with pain, burning, numbness, and paraesthesia of the heel or feet, with or without ulcers, were evaluated. All patients were tested for fasting and postprandial blood sugar levels and HbA1c. Clinically, all patients were evaluated through a three-point sensory testing using Semmes-Weinstein 10 g filaments (measured on a scale of 0 to 10 at a minimum of four sites: heel, toe, lateral malleolus, and medial malleolus), Tinel’s sign at the tarsal tunnel, and the presence of ulcers on the foot. All patients underwent Nerve Conduction Velocity (NCV) studies performed by a neurologist at this hospital, which included assessments of distal motor and sensory latency and Sensory Nerve Action Potential (SNAP). Imaging studies in the form of MRI were conducted for a complete work-up of these patients and further diagnosis of TTS.
Statistical Analysis
All demographic data, clinical examination results, nerve conduction study results, and MRI findings were recorded in an MS Excel spreadsheet. The data were coded and analysed using Statistical Package for Social Sciences (SPSS) version 23.0 (IBM Corp). Descriptive statistics were generated using means/standard deviations and medians/Interquartile Ranges (IQRs) for continuous variables, and frequencies and percentages for categorical variables. Group comparisons were performed using the Chi-square test. Sensitivity of the test was calculated by dividing the number of true positives by the sum of true positives and false negatives. Specificity of the test was calculated by dividing the number of true negatives by the sum of true negatives and false positives. Statistical significance was set at a p-value of 0.05.
Results
The mean age of presentation was 61.4±11.2 years, and the median age was 59 years. Of the 30 patients enrolled, 20 were males (66.7%) and 10 were females (33.3%) [Table/Fig-1].
Age-wise and gender-wise demographic data of patients.
| Parameters | n (%) |
|---|
| Age (years) |
| ≤50 | 3 (10.0) |
| 51-60 | 13 (43.3) |
| 61-70 | 8 (26.7) |
| 71-80 | 6 (20.0) |
| Mean±SD (Range) | 61.4±11.2 (43-77) |
| Median (IQR) | 59 (53-69) |
| Gender |
| Male | 20 (66.7) |
| Female | 10 (33.3) |
The mean fasting blood sugar and postprandial levels were 163.7±78 mg/dL and 228.5±84.3 mg/dL, respectively. The mean HbA1c level was 8.5±1.04% [Table/Fig-2]. The incidence of TTS in diabetic patients was found to be 14 (46.7%) electrodiagnostically, 22 (73.3%) clinically, and 25 (83.3%) radiologically.
Blood sugar levels of patients included in the study.
| Blood sugar(mg/dL) | n (%) |
|---|
| Fasting | <100 | 2 (6.7) |
| ≥100 | 28 (93.3) |
| Mean±SD (Range) | 163.7±78.0 (74-367) |
| Median (IQR) | 148 (112-175) |
| Post Prandial (PP) | <200 | 19 (63.3) |
| ≥200 | 11 (36.7) |
| Mean±SD (Range) | 228.5±84.3 (118-430) |
| Median (IQR) | 224 (146-274) |
| HbA1c (%) |
| Mean±SD (Range) | 8.5±1.04 (7.1-10.5) |
| Median (IQR) | 8.3 (8.0-9.5) |
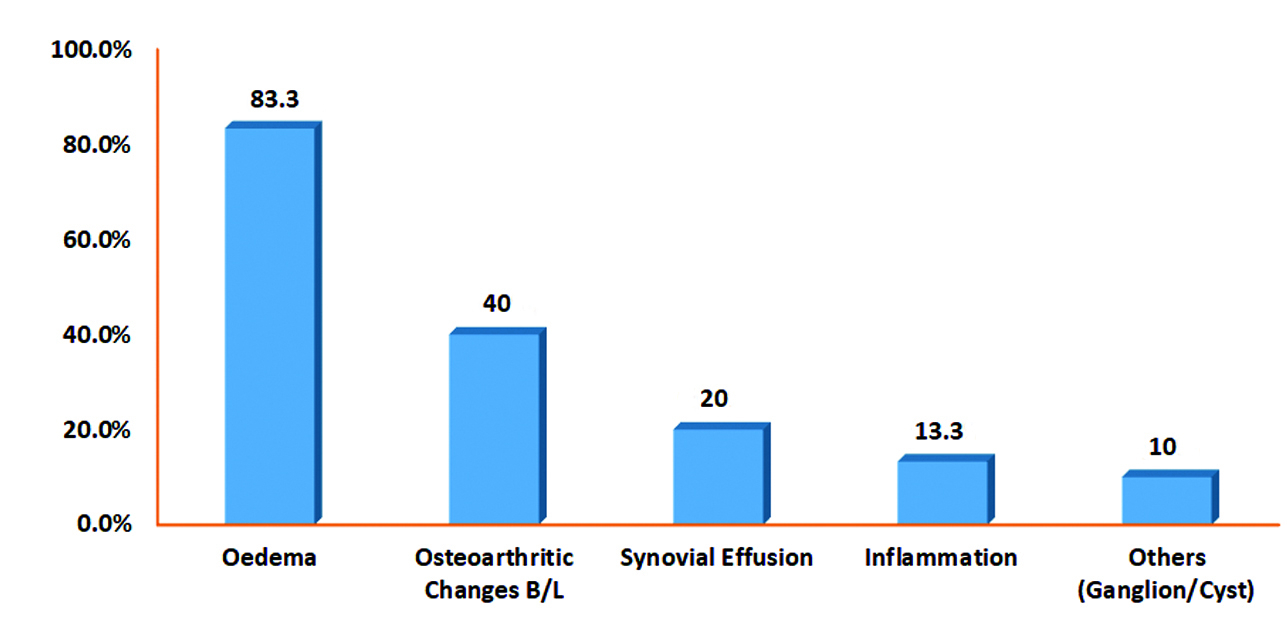
The most common finding in the imaging conducted was oedema, which was observed in 25 (83.3%) patients. Inflammation and ganglion cysts were seen in 4 (13.3%) and 3 (10%) patients, respectively [Table/Fig-3].
MRI findings of the patients.
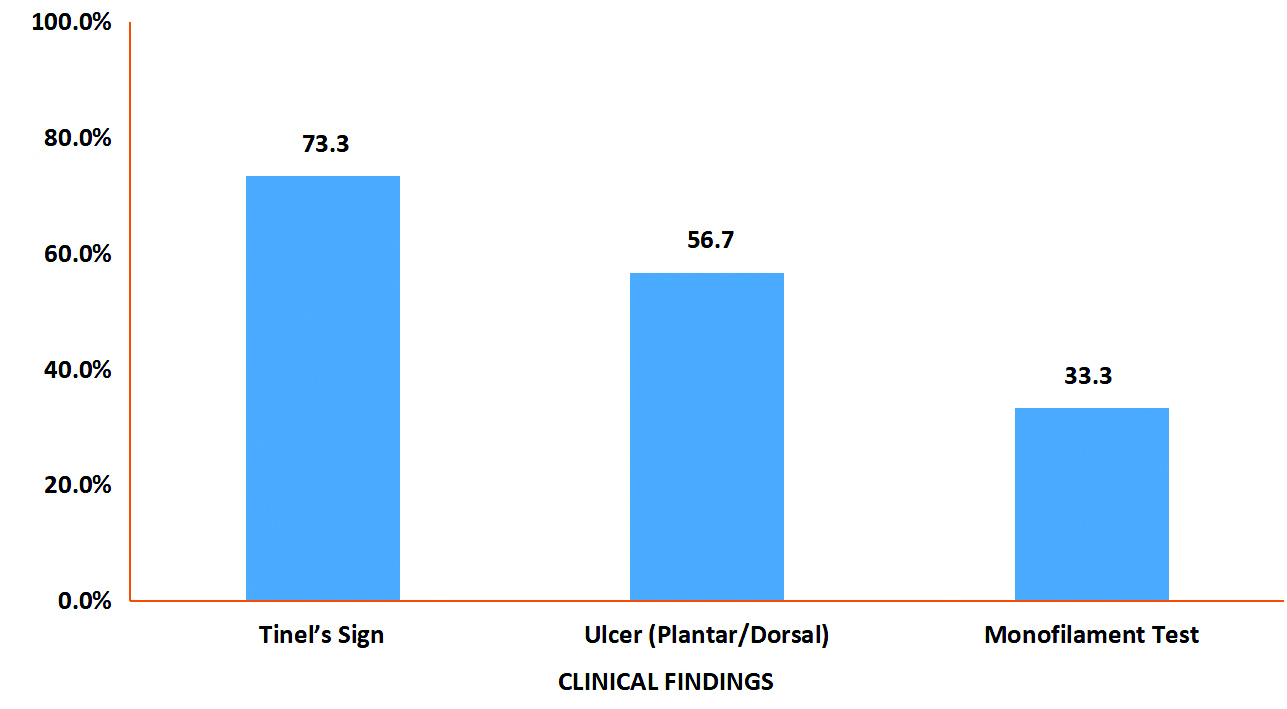
Tinel’s sign was positive in 22 (73.3%) of patients. Additionally, 10 (33.3%) of patients exhibited a loss of sensation when measured on a scale of 10 at a minimum of four sites (heel, toe, lateral malleolus, and medial malleolus). Non healing diabetic ulcers were present in 17 (56.7%) of patients [Table/Fig-4].
Findings of clinical evaluation of patients.
On electrodiagnostic studies, the motor amplitude was not recordable in 10 (33.3%) of patients. The H reflex was not recordable in 21 (70%) of patients, and the sensory amplitude was not recordable in 26 (86.7%) of patients [Table/Fig-5].
Findings of electrodiagnostic studies.
| NCV findings | n (%) |
|---|
| Motor amplitude | Not recordable | 10 (33.3) |
| Reduced amplitude and increasedlatency | 14 (46.7) |
| Normal | 6 (20.0) |
| F wave latency | Not recordable | 19 (63.3) |
| Increased latency | 11 (36.7) |
| Normal | 0 |
| H reflex | Not recordable | 21 (70) |
| Increased latency | 6 (20) |
| Normal | 3 (10.0) |
| Sensory | Not recordable | 26 (86.7) |
| Reduced amplitude and velocity with increased latency | 0 |
| Normal | 4 (13.3) |
The positivity of Tinel’s sign was significantly associated with electrodiagnostic confirmation of TTS (p-value=0.0001). The monofilament test was also significantly associated with electrodiagnostic confirmation of TTS (p-value=0.039) [Table/Fig-6].
Association between clinical findings and electro diagnostic findings.
| Clinical findings | Electrodiagnostic findings | Total | Chi-square/t-value | p-value |
|---|
| TTS | No TTS |
|---|
| Tinel’s sign | 14 (100%) | 8 (16.7%) | 22 (80%) | 15.093 | 0.0001* |
| Ulcer (Plantar/Dorsal) | 9 (58.3%) | 8 (50%) | 17 (56.7%) | 0.136 | 0.713 |
| Monofilament test | 8 (57.14%) | 2 (12.5%) | 10 (33.3%) | 8.438 | 0.039 |
*p-value <0.05; statistically significant
Clinical findings were confirmed by electrodiagnostic findings in 17 (56.7%) of patients. Radiological confirmation of TTS was corroborated by electrodiagnostic studies in 15 (50%) of patients. The clinical findings of TTS were confirmed by radiological findings in 80% of patients [Table/Fig-7].
Diagnostic association of clinical evaluation, radiological findings with electrodiagnostic studies and clinical findings with radiological studies.
| Findings | Electrodiagnostic findings | Total |
|---|
| TTS | No TTS |
|---|
| Clinical findings | TTS | 11 | 6 | 17 (56.7) |
| No TTS | 3 | 10 | 13 (43.3) |
| Total | 14 (46.7%) | 16 (53.3%) | 30 (100.0) |
| Radiological findings | TTS | 13 | 2 | 15 (50.0) |
| No TTS | 1 | 14 | 15 (50.0) |
| Total | 14 (46.7%) | 16 (53.3%) | 30 (100.0) |
| Findings | Radiological findings | Total |
| TTS | No TTS |
| Clinical findings | TTS | 21 | 3 | 24 (80) |
| No TTS | 3 | 3 | 6 (20) |
| Total | 24 (80%) | 6 (20%) | 30 (100) |
TTS: Tarsal tunnel syndrome
The sensitivity of the association between clinical findings and electrodiagnostic studies was 78.57%, with a specificity of 62.5%. The sensitivity of the association between radiological findings and electrodiagnostic studies was 92.86%, with a specificity of 87.5%. The sensitivity of the association between clinical findings and radiological studies was 87.5%, with a specificity of 50% [Table/Fig-8].
Sensitivity and specificity of association of clinical findings, radiological findings with electrodiagnostic studies and clinical findings with radiological studies.
| Statistic | Value | 95% CI |
|---|
| Association of clinical findings with electrodiagnostic studies | Sensitivity | 78.57% | 49.20% to 95.34% |
| Specificity | 62.50% | 35.43% to 84.80% |
| Positive predictive value | 64.71% | 47.92% to 78.50% |
| Negative predictive value | 76.92% | 53.28% to 90.69% |
| Accuracy | 70.00% | 50.60% to 85.27% |
| Association of radiological findings with electrodiagnostic studies | Sensitivity | 92.86% | 66.13% to 99.82% |
| Specificity | 87.50% | 61.65% to 98.45% |
| Positive predictive value | 86.67% | 63.81% to 95.99% |
| Negative predictive value | 93.33% | 67.73% to 98.94% |
| Accuracy | 90.00% | 73.47% to 97.89% |
| Association of clinical findings with radiological studies | Sensitivity | 87.5% | 59.54% to 98.34% |
| Specificity | 50% | 44.90% to 92.21% |
| Positive predictive value | 87.5% | 57.84% to 88.50% |
| Negative predictive value | 50% | 59.36% to 95.39% |
| Accuracy | 80.00% | 61.43% to 92.29% |
CI: Confidence interval
Discussion
In this study, the mean age of presentation was 61.4±11.2 years, with a male predominance (66.7%). A similar study by Rinkel WD et al., which examined 113 patients in the Netherlands, reported a comparable mean age of 62.7 years [10].
Examination also included Tinel’s sign, which involves light tapping over the nerve to elicit a sensation of tingling or “pins and needles” along the distribution of the nerve. It is considered positive when the tingling or prickling sensation is felt. This sign was found to be positive in 73.3% of patients, with a significant p-value of 0.0001. Cimino WR found this in all 97 patients in their review of the literature [11]. Linscheid RL et al., found that Tinel’s sign was positive in 24 out of 34 patients in their study conducted in Atlanta [12].
MRI findings suggestive of oedema were the main reason for compression of the common peroneal nerve and tibial nerve in the tarsal tunnel. Oedema was the predominant finding present in 25 patients (83.3%). Jung HJ et al., conducted a study on 33 patients in Incheon, Korea, and suggested that MRI is excellent for demonstrating musculotendinous and neurovascular structures [8]. MRI can clearly reveal the anatomy of the tarsal tunnel and its contents, along with the presence or extent of space-occupying lesions leading to TTS [13]. Kerr R and Frey C found that oedema was the main factor associated with inflammation of the tibialis posterior tendon within the tunnel [14].
Sensory nerve conduction studies were more sensitive and yielded more convincing diagnostic findings. Approximately 26 patients (86.7%) had no recordable potentials, while four patients (13.3%) had normal findings. Patel AT et al., found that SNAP were absent in 92.8% of patients with TTS, while mixed nerve conduction studies were abnormal in 85.7% of patients [15]. Sodani A et al., conducted a study on 40 patients in India and found that motor nerve conduction studies were significantly abnormal in patients with TTS [16]. These findings are quite comparable to those of present study.
This was a single-centre study. All three modalities for diagnosing TTS-clinical, radiological, and electrodiagnostic studies-were used in this study. Longer follow-up durations and further studies are needed to ascertain the impact of these factors on long-term complications in type II diabetic patients.
Limitation(s)
The study had a small sample size. Patients with diabetic foot at risk who had a low Wagner’s grade were included in the study, while those with a higher Wagner’s grade were excluded.
Conclusion(s)
The diagnosis of TTS can be challenging. It is primarily based on clinical examinations such as the monofilament test, Tinel’s sign, and the assessment of diabetic ulcers. To further support the diagnosis, imaging modalities like MRI can be useful for evaluating the depth of the tunnel and the status of the underlying structures, including oedema, inflammation, and compression due to synovial fluid effusion or ganglia. Additionally, electrodiagnostic studies can help confirm the diagnosis.
*p-value <0.05; statistically significant
TTS: Tarsal tunnel syndrome
CI: Confidence interval
Authors’ contribution:
Study conception and design was done by VK and MAP. Data collection was done by VK, SD, SC, RM and AK. Analysis and interpretation of results was done by PD and VK. Draft manuscript preparation was done by VK and SM. All authors reviewed the results and approved the final version of the manuscript.
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 31, 2024
Manual Googling: May 20, 2024
iThenticate Software: Sep 03, 2024 (10%)
[1]. Narayan S, Goel A, Singh AK, Thacker AK, Singh N, Gutch M, High resolution ultrasonography of peripheral nerves in diabetic patients to evaluate nerve cross sectional area with clinical profileBr J Radiol 2021 94(1121):2020017310.1259/bjr.2020017333733810 [Google Scholar] [CrossRef] [PubMed]
[2]. Mehraj DrM, A review of Wagner classification and current concepts in management of diabetic footInt J Orthop Sci 2018 4(1n):933-35.10.22271/ortho.2018.v4.i1n.133 [Google Scholar] [CrossRef]
[3]. Boulton AJ, Armstrong DG, Albert SF, American Diabetes Association; American Association of Clinical EndocrinologistsComprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical EndocrinologistsDiabetes Care 2008 31:1679-85.10.2337/dc08-902118663232 [Google Scholar] [CrossRef] [PubMed]
[4]. Ormeci T, Mahirogulları M, Aysal F, Tarsal tunnel syndrome masked by painful diabetic polyneuropathyInt J Surg Case Rep 2015 15:103-06.10.1016/j.ijscr.2015.08.03326333036 [Google Scholar] [CrossRef] [PubMed]
[5]. Padua L, Cuccagna C, Giovannini S, Coraci D, Pelosi L, Loreti C, Carpal tunnel syndrome: Updated evidence and new questionsLancet Neurol 2023 22(3):255-67.10.1016/S1474-4422(22)00432-X36525982 [Google Scholar] [CrossRef] [PubMed]
[6]. Pendsey S, Abbas ZG, The step-by-step program for reducing diabetic foot problems: A model for the developing worldCurr Diab Rep 2007 7(6):425-28.10.1007/s11892-007-0071-x18255004 [Google Scholar] [CrossRef] [PubMed]
[7]. Dube S, Hulke SM, Wakode SL, Khadanga S, Thakare AE, Bharshankar RN, Effectiveness of Semmes Weinstein 10 gm monofilament in diabetic peripheral neuropathy taking nerve conduction and autonomic function study as reference testsJ Fam Med Prim Care 2022 11(10):6204-08.10.4103/jfmpc.jfmpc_195_2236618173 [Google Scholar] [CrossRef] [PubMed]
[8]. Jung HJ, Lee SW, Jeong YM, Choi HY, Kim HS, Park HG, The usefulness of the preoperative magnetic resonance imaging findings in the evaluation of tarsal tunnel syndromeJ Korean Soc Radiol 2012 66(2):18310.3348/jksr.2012.66.2.183 [Google Scholar] [CrossRef]
[9]. Bansal V, Kalita J, Misra UK, Diabetic neuropathyPostgrad Med J 2006 82(964):95-100.10.1136/pgmj.2005.03613716461471 [Google Scholar] [CrossRef] [PubMed]
[10]. Rinkel WD, Castro Cabezas M, Birnie E, Coert JH, The natural history of tarsal tunnel syndrome in diabetic subjectsJ Plast Reconstr Aesthet Surg 2020 73(8):1482-89.10.1016/j.bjps.2020.02.03332276769 [Google Scholar] [CrossRef] [PubMed]
[11]. Cimino WR, Tarsal tunnel syndrome: Review of the literatureFoot Ankle 1990 11(1):47-52.10.1177/1071100790011001102210534 [Google Scholar] [CrossRef] [PubMed]
[12]. Linscheid RL, Burton RC, Fredericks EJ, Tarsal-tunnel syndromeSouth Med J 1970 63(11):1313-23.10.1097/00007611-197011000-000264319887 [Google Scholar] [CrossRef] [PubMed]
[13]. Frey C, Kerr R, Magnetic resonance imaging and the evaluation of Tarsal tunnel syndromeFoot Ankle 1993 14(3):159-64.10.1177/1071100793014003098491431 [Google Scholar] [CrossRef] [PubMed]
[14]. Kerr R, Frey C, MR Imaging in Tarsal tunnel syndromeJ Comput Assist Tomogr 1991 15(2):280-86.10.1097/00004728-199103000-000181672132 [Google Scholar] [CrossRef] [PubMed]
[15]. Patel AT, Gaines K, Malamut R, Park TA, Toro DRD, Holland N, Usefulness of electrodiagnostic techniques in the evaluation of suspected tarsal tunnel syndrome: An evidence-based reviewMuscle Nerve 2005 32(2):236-40.10.1002/mus.2039316003732 [Google Scholar] [CrossRef] [PubMed]
[16]. Sodani A, Dube M, Jain R, Value of motor nerve conduction studies in the diagnosis of idiopathic tarsal tunnel syndrome: A single-center prospective observational study from IndiaAnn Indian Acad Neurol 2018 21(1):35-41.10.4103/aian.AIAN_320_1729720796 [Google Scholar] [CrossRef] [PubMed]