Patients with diabetes and CKD have an increased risk of all-cause mortality, cardiovascular mortality and kidney failure. The natural course of DKD progression includes glomerular hyperfiltration, followed by progressive albuminuria, declining Glomerular Filtration Rate (GFR) and ultimately ESRD. Findings of glomerular hypertrophy, glomerulosclerosis and tubulointerstitial inflammation and fibrosis are associated with the metabolic changes in diabetes. The risk of onset and progression of DKD persists despite current diabetes therapies. Therefore, there is an urgent need to improve health outcomes for patients with DKD. To achieve this, it is crucial to identify the disease at an early stage and develop therapeutic agents targeting kidney-specific disease mechanisms, such as glomerular hyperfiltration, inflammation and fibrosis [4].
The clinical factors influencing the prediction of the progression of CKD to ESRD in DN include the duration of diabetes, blood pressure, estimated Glomerular Filtration Rate (eGFR), proteinuria and glycated haemoglobin a1c level [5]. However, DN affects all structural components of the kidney and manifests with diverse pathological findings. Therefore, recognising such lesions and their morphological characteristics via renal biopsy may help in preventing, slowing down, or even reversing the processes of DN [6].
In diabetic patients, the results of renal biopsy can be classified into DN, Non Diabetic Renal Disease (NDRD), or DN with NDRD (mixed forms). Renal biopsy in DN, especially in patients undergoing new treatments, may play a role in assessing renal protection or regression of diabetic histological lesions. Additionally, it has been observed that early diagnosis and subsequent treatment of NDRD in diabetic patients have led to better prognosis [7].
To aid DN patients, it is important to identify prognostic factors. The Renal Pathological Society has indicated that Glomerular Basement Membrane (GBM) thickening, mesangial expansion, nodular sclerosis and advanced diabetic glomerulosclerosis are key features. A score is calculated based on tubulointerstitial and vascular lesions, which include IFTA, Arteriolar Hyalinosis (AH), inflammatory interstitial infiltrates, the presence of large vessels and arteriosclerosis [8]. Furthermore, renal IFTA is one of the primary endpoints of kidney injury and its accurate quantification in biopsy samples aids in establishing the diagnosis and assessing the severity of the disease [9]. The severity of IFTA has been noted to be associated with renal events and mortality in patients with type 2 diabetes and biopsy-proven DN [10]. Additionally, obsolescent glomerulosclerosis is recognised as another prognostic factor in DN, as highlighted in previous literature [11].
Data on renal biopsy, particularly in the current study setting, is very limited. The present study contributes to the existing literature by addressing the gap regarding the magnitude of these findings and by providing data on pathological markers such as IFTA and obsolescent glomerulosclerosis [5,12]. Therefore, the present study was conducted to describe the renal biopsy profiles among the subjects and to determine the significance of the number of obsolescent glomeruli and IFTA scoring in predicting DKD and its severity.
Materials and Methods
The present study was a cross-sectional study conducted over a period of three years at an urban tertiary care hospital in the Department of General Medicine and Nephrology at Kempegowda Institute of Medical Sciences Hospital and Research Centre, Bengaluru, Karnataka, India, from 2019 to 2022, involving a total of 189 patients selected through purposive sampling. Ethical clearance was obtained from the Institutional Ethics Committee (KIMS/IEC/A150/M/2024).
Inclusion and Exclusion criteria: The study included patients with diabetes mellitus who presented with renal disease and required a renal biopsy to confirm the diagnosis. These patients were admitted to the study Institute. Patients who were unwilling to participate in the study were excluded.
Sample size calculation: Considering 34.4% prevalence of CKD stage 3 among type 2 diabetic patients in India [13] and an additional 7% for inadequate sampling, the sample size of 189 was estimated with 5% alpha error, 7% absolute precision by applying the formula n= zα/2 2 pq/l2 where n- sample size, p-prevalence of DKD, q=1-p, l-precision (or margin of error), zα/2- 1.96 for alpha 0.05.
Study Procedure
After obtaining written informed consent from all the study participants, details regarding the socio-demographic data and clinical history were collected using a semi-structured questionnaire by interview technique. Clinical examination included vitals, general physical examination and systemic examination were done.
A renal biopsy was performed under real-time ultrasonography guidance by nephrologists. The indications for the renal biopsy included proteinuria of more than 0.5 g/day or atypical DN, such as renal involvement without diabetic retinopathy and/or the presence of urinary Red Blood Cells (RBCs). Renal tissue was obtained through needle biopsy and specimens were processed for light microscopy, immunofluorescence and Electron Microscopy (EM). A biopsy core was labelled as an adequate sample if it contained five glomeruli for glomerular lesions and ten glomeruli in cases of tubulointerstitial disease. Biopsy samples with an inadequate number of glomeruli for either light microscopy or immunofluorescence studies were excluded from the analysis. Transplant kidney biopsies were also excluded from the study. The biopsy report was obtained and analysed for the profile [14]. The severity of lesions was graded into four classes and IFTA scores were noted, along with the number of glomeruli and obsolescent glomeruli [15].
Operational Definition
Classes of Diabetic Nephropathy (DN): Based on the glomerular involvement, the severity of lesions is graded into four classes. Class I is characterised by normal optical microscopy and basal glomerular thickening observed in EM. Class II is characterised by mesangial expansion and is subdivided into Class IIa and Class IIb according to the severity of this lesion. Class III is characterised by the presence of at least one nodular lesion (Kimmelstiel-Wilson lesion), provided that no more than 50% of the glomeruli are sclerosed. Class IV, or advanced diabetic glomerulosclerosis, designates biopsies with more than 50% glomerulosclerosis when this lesion can be attributed to DN, specifically the presence of Class II or III lesions, or a long history of diabetes along with diabetic retinopathy [6].
Pure Non Diabetic Kidney Disease (NDKD): This was defined by the presence of predominant vasculopathy, interstitial fibrosis, tubular atrophy and/or specific glomerular changes in the absence of classical changes associated with DKD [12].
Interstitial Fibrosis and Tubular Atrophy (IFTA) scores: These scores are evaluated using a semi-quantitative scale of 0-3+, reflecting the percentage of the total involved area of interstitium and tubules as follows: 0 for absence of interstitial fibrosis, 1 for <25%, 2 for 25%-50% and 3 for >50% [15].
Obsolescent glomerulosclerosis is characterised by ischaemic obsolescent glomerulosclerosis, which presents as a retracted glomerular tuft surrounded by a hypocellular homogeneous collagen matrix beginning at the vascular pole adjacent to the glomerular stalk [11].
The terms DN, DKD, NDRD and NDKD are used interchangeably in the current study.
Statistical Analysis
The data were entered into Microsoft Excel and analysed using SPSS version 20.0. Categorical data were presented as proportions, while continuous data were expressed as mean±SD or median with range, depending on whether the data followed a parametric or non parametric distribution. Categorical variables were analysed using the Chi-square test and continuous non parametric variables were compared between groups using the Mann-Whitney U test. The strength of association was expressed using odds ratios, calculated through bivariate logistic regression. The correlation between ordinal data, specifically severity of DKD, IFTA scores and the number of obsolescent glomeruli was analysed using Spearman’s correlation coefficient. A p-value of less than 0.05 was considered statistically significant.
Results
A total of 189 study subjects were included in the analysis. The majority, 114 (60.3%), were aged between 41 to 60 years, with a mean age of 53.02±11.00 years, ranging from 21 to 81 years. Among the participants, 148 (78.3%) were males. Based on the renal biopsy findings, DN was the most common diagnosis, found in 127 (67.6%) subjects, while the remaining 61 (32.4%) had NDKD [Table/Fig-1].
Distribution of study subjects based on socio-demographic and clinical details.
| Variables | Frequency (n) | Percentage (%) |
|---|
| Age-group in years (N=189) |
| ≤40 | 024 | 12.7 |
| 41-60 | 114 | 60.3 |
| >60 | 051 | 27.0 |
| Mean age±SD (years) | 53.02±11.00 |
| Sex (n=189) |
| Males | 148 | 78.3 |
| Females | 041 | 21.7 |
| Renal biopsies (n=188)¥ |
| Diabetic Nephropathy (DN) | 127 | 67.6 |
| Non Diabetic Kidney Disease (NDKD) | 061 | 32.4 |
¥One has been excluded as it had inadequate sample
Among those with DN, 63 (49.6%) had Class IV chronic DN, followed by Class III in 54 (42.5%) cases, Class IIb in 6 (4.7%), Class IIa in 2 (1.6%) and Class I in 2 (1.6%) [Table/Fig-2].
Distribution of study subjects based on histopathological findings in Diabetic Nephropathy (DN).
| Histopathological findings in diabetic nephropathy (DN) (n=127) | Frequency (n) | Percentage (%) |
|---|
| Class I | 02 | 1.6 |
| Class IIa | 02 | 1.6 |
| Class IIb | 06 | 4.7 |
| Class III | 54 | 42.5 |
| Class IV | 63 | 49.6 |
Of those with NDKD, chronic interstitial nephritis was the most common diagnosis, found in 15 (24.6%) subjects, followed by acute tubular injury and membranous glomerulonephritis, each present in 7 (11.5%) subjects. Chronic glomerulosclerosis and focal segmental glomerulosclerosis were found in 6 (9.8%) subjects each [Table/Fig-3].
Distribution of study subjects based on histopathological findings in Non Diabetic Kidney Disease (NDKD).
| Histopathological findings in Non Diabetic Kidney Disease (NDKD) (n=61) | Frequency (n) | Percentage (%) |
|---|
| Chronic interstitial nephritis | 15 | 24.6 |
| Acute tubular injury | 07 | 11.5 |
| Membranous glomerulonephritis | 07 | 11.5 |
| Chronic glomerulosclerosis | 06 | 9.8 |
| Focal segmental glomerulosclerosis | 06 | 9.8 |
| Immunoglobulin A (IgA) nephropathy | 05 | 8.2 |
| Acute interstitial nephritis | 04 | 6.6 |
| Minimal change disease | 03 | 4.9 |
| Acute tubular interstitial nephritis | 03 | 4.9 |
| Membranoproliferative glomerulonephritis | 03 | 4.9 |
| Cast nephropathy | 02 | 3.3 |
| Pyelonephritis | 02 | 3.3 |
| Acute on chronic interstitial nephritis | 02 | 3.3 |
| Hypertensive nephrosclerosis | 02 | 3.3 |
| Chronic glomerulonephritis | 01 | 1.6 |
| Mesangial proliferative glomerulonephritis | 01 | 1.6 |
| C3 glomerulonephritis | 01 | 1.6 |
| Hyperfiltration injury | 01 | 1.6 |
n>61 and % >100 because multiple co-existing findings
The proportion of subjects with DKD increased with rising IFTA scores, from 11 (20.4%) to 51 (91.1%). However, the proportions of individuals with IFTA scores of 2 and 3 remained nearly equal. Notably, IFTA scoring was significantly associated with DKD compared to NDKD (p<0.005) [Table/Fig-4].
Distribution of study subjects based on IFTA scoring among Diabetic (DKD) and Non Diabetic Kidney Disease (NDKD).
| IFTA scoring | Diabetic Kidney Disease (DKD) | Non Diabetic Kidney Disease (NDKD) | χ2-value(p-value) |
|---|
| 0 | 11 (20.4) | 43 (79.6) | 77.91 (<0.005)* |
| 1 | 47 (82.5) | 10 (17.5) |
| 2 | 51 (91.1) | 05 (8.9) |
| 3 | 18 (85.7) | 03 (14.3) |
*indicates statistically significant association at p<0.05
The median number of obsolescent glomeruli (6 vs 2) and IFTA scores (2 vs 0) were significantly higher in chronic DN compared to NDKD (p<0.001). However, the number of glomeruli did not differ significantly between the two groups (p=0.38) [Table/Fig-5].
Comparison of IFTA scores in Chronic Diabetic Nephropathy (DN) and Non Diabetic Kidney Disease (NDKD).
| Variables | Median (Range) | Mann-Whitney U value (p-value) |
|---|
| DN | NDKD |
|---|
| No. of glomeruli | 14 (1 to 55) | 15 (1 to 64) | 3565.5(0.38) |
| No. of obsolescent glomeruli | 06 (0 to 45) | 02 (0 to 34) | 2522.5(<0.001)* |
| IFTA scores | 02 (0 to 3) | 00 (0 to 3) | 1353.0(<0.001)* |
*indicates statistically significant difference at p<0.05
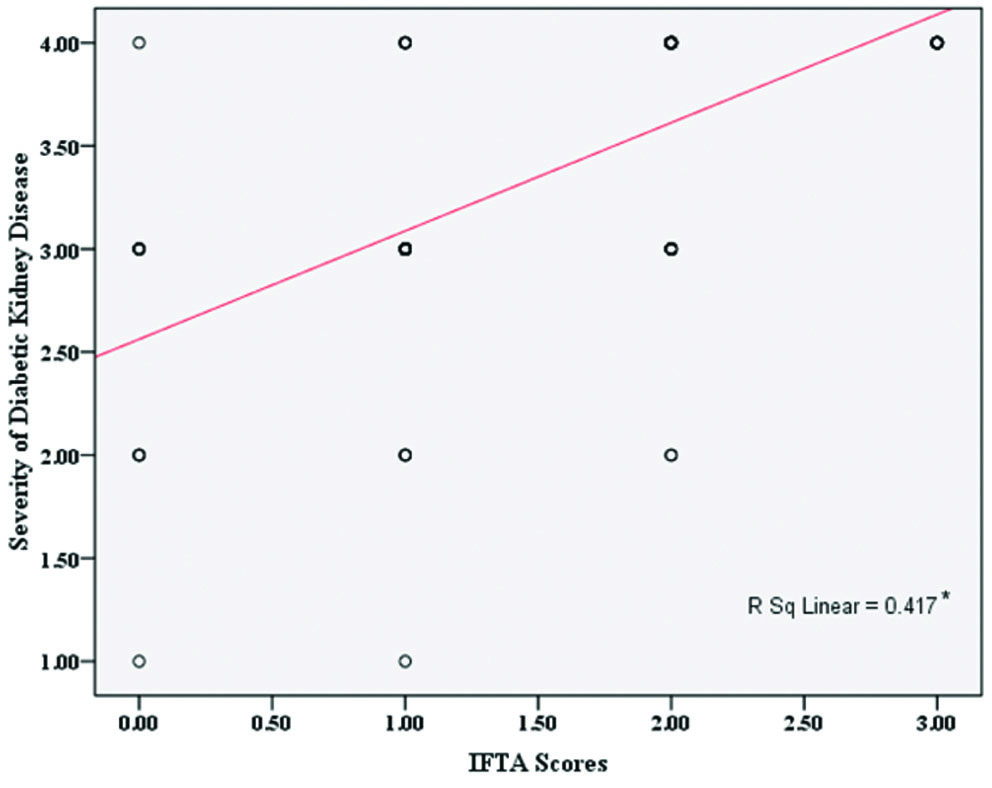
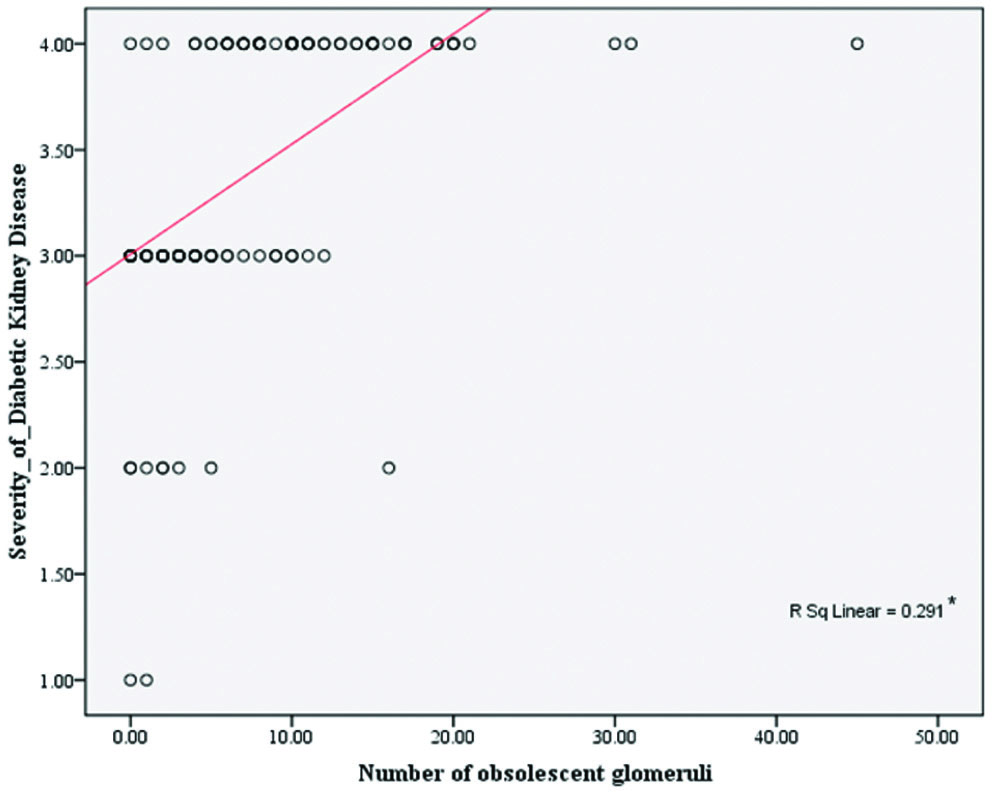
There was a significant correlation between the severity of DKD and IFTA scores, indicating that IFTA scores significantly increase with the severity of DKD (r=0.7; p<0.001), where the r value indicates a high positive correlation [Table/Fig-6]. Similarly, significant positive correlations were established between the number of obsolescent glomeruli and the severity of DKD (r=0.7; p<0.05) [Table/Fig-7].
Correlation between severity of Diabetic Kidney Disease (DKD) and IFTA Scores.
*indicates significant statistical correlation at p<0.05
Correlation between severity of Diabetic Kidney Disease (DKD) and number of obsolescent glomeruli.
*indicates significant statistical correlation at p<0.05
Among the DN patients, the median IFTA scores and the median number of obsolescent glomeruli in those with severe DKD (Class III and Class IV) were 2 and 7, respectively, while in those with Class I and II, they were 1 and 1.5, respectively. These differences were statistically significant (p=0.003). The odds ratio of 4.32 suggests that for each unit increase in the IFTA score, there was a 4.32-fold higher risk of having severe DKD (Class III and Class IV) compared to those with less severe forms (Class I and II). Similarly, a unit increase in the number of obsolescent glomeruli increased the risk of having severe DKD (Class III and Class IV) by 1.24 times compared to those with less severe forms (Class I and II), respectively (p=0.003) [Table/Fig-8].
Predictive ability of IFTA scores and number of obsolescent glomeruli in severity of Chronic Diabetic Nephropathy (DN).
| Predictive variables | Median (Range) | Mann-Whitney U value (p-value) | OR (95% CI) |
|---|
| Class I/II | Class III/IV |
|---|
| IFTA scores | 01 (0 to 2) | 02 (0 to 3) | 277.00 (0.003)* | 4.32* (1.60 to 11.63) |
| Number of obsolescent glomeruli | 1.5 (0 to 16) | 07 (0 to 45) | 268.50 (0.003)* | 1.24 (1.02 to 1.52) |
*indicates statistical significance at p<0.05
Discussion
In the current study, nearly two-thirds of diabetics had DN and most of the subjects with DN had Class IV and Class III chronic DN. The number of obsolescent glomeruli and IFTA scores were found to be independent predictors of the severity of DN.
India is one of the three countries with the highest burden of CKD due to DM [16]. Although renal involvement in diabetes is primarily due to DN, a considerable proportion of patients undergoing kidney biopsy have been noted to exhibit NDKD, which can present alone or superimposed on DKD [4,17,18]. Given the high burden of diabetes in India, both DKD and NDKD are expected to be prevalent [12]. There are significant therapeutic and prognostic implications in diagnosing NDKD in diabetic patients [17].
Previously conducted research has reported severe glomerular injury, an increased degree of interstitial inflammation, severe IFTA and a higher occurrence of arterial hypertension and arteriosclerosis, all of which are associated with renal endpoints indicating renal damage [19,20]. The critical role of these factors in the progression of DN has also been established in other studies [20]. Similarly, the occurrence of IFTA has also been noted in NDKD [12]. Obsolescent glomeruli appeared to be significantly associated with end-stage renal disease in patients with type 2 diabetes but did not retain its significance after adjusting for confounders [11].
In addition to this background, data on renal biopsy findings and the pathological features that have prognostic utility in DN are limited [5,12]. Hence, this study was conducted to assess the histopathological features of patients who underwent renal biopsy [18] and to determine the association of IFTA scores with DKD and its severity.
Tong X et al., reported the prevalence rate of DN in different studies ranging from 8.2% to 62.7%, with an average of 41.3%. Similarly, DN was the most common diagnosis found in 67.6% of the study participants [21]. Prakash J et al., reported the mean age of study subjects to be around 52 years, with the majority being male. In their study, isolated DN was found in 52% of cases and DN with superimposed NDKD was present in 20%. The present study found a mean age of around 53 years, with the majority being male and 67.6% of participants had DN [22].
Sahay M et al., noted that the most common histological class was Class IV, observed in 43.02% of cases, followed by Class III DN in 27.90% of cases. Class IIa and Class IIb were each found in 12.79% of cases, while Class I DN was present in 3.48% of cases [23]. Zajjari Y et al., found Class III to be the most common histological class among DN cases, accounting for 42.3% [24]. However, the present study findings were consistent with those of Sahay M et al., showing that nearly 50.0% had Class IV chronic DN, followed by Class III (42.5%), Class IIb (4.7%), Class IIa (1.6%) and Class I (1.6%) [23]. The current findings slightly differed from those of Zajjari Y et al., as the severity of DN manifestations depends on the chronicity of diabetes and glycemic control [24,25].
Tolani P et al., found NDKD in 48.15% of cases, with IgA nephropathy being the most common, followed by membranous glomerulopathy, focal segmental glomerulosclerosis and other conditions such as tubulointerstitial diseases and crescentic glomerulonephritis [14]. In contrast, this study observed NDKD in 32.4% of cases, with chronic interstitial nephritis being the most prevalent, followed by acute tubular injury, membranous glomerulonephritis, chronic glomerulosclerosis, focal segmental glomerulosclerosis and IgA nephropathy. Acute interstitial nephritis and other diseases such as minimal change disease, acute tubular interstitial nephritis, membranoproliferative glomerulonephritis, cast nephropathy, pyelonephritis, acute on chronic interstitial nephritis, hypertensive nephrosclerosis, chronic glomerulonephritis, mesangial proliferative glomerulonephritis and C3 glomerulonephritis accounted for less than 5% of cases. The differences in the types of NDKD may be attributed to varying study settings and the duration of diabetes [14].
Zajjari Y et al., did not show any significant difference in the number of glomeruli across different classes of DN, with means of 14 and 15, indicating optimal quality of the biopsy samples [24]. As explained above, a study by Zhao L et al., found that obsolescent glomeruli were significantly associated with end-stage renal disease in patients with type 2 diabetes; however, this significance did not hold after adjusting for confounders [11]. These findings align with the current study, although the loss of significance and the observed differences may be attributed to variations in the study settings. Zhao L et al., also reported a 1.24 times higher risk of having Class III and IV DN compared to those with Class I and II DN.
As IFTA scores increased, the proportion of subjects with DKD rose until IFTA score 2, after which the proportion remained relatively stable, with a slight decrease noted at IFTA score 3. The scores were significantly associated with DKD and IFTA scores (2 vs. 0) were significantly higher in chronic DN compared to NDKD in this study. A significant positive correlation between IFTA scores and the grade of DN was also observed in the current study, consistent with findings from other studies conducted by Kim T et al., Shimizu M et al., and Zajjari Y et al., which demonstrated associations and positive correlations with CKD stage, as well as higher mean values in Class IV DN [8,10,24]. Additionally, this study highlighted the increased risk of higher severity with rising IFTA scores, showing a 4.32 times higher risk of having severe DKD (Class III and Class IV) compared to those with less severe forms (Class I and II).
Limitation(s)
The study was limited to a single setting and purposive sampling was used due to logistical constraints and the nature of the investigation.
Conclusion(s)
The data indicate that DN contributed to nearly two-thirds of the individuals with diabetes, while one-third had NDKD. More than 90% of those with DN had Class IV and Class III chronic DN. Among those with NDKD, nearly 50% exhibited pathological findings of chronic interstitial nephritis, acute tubular injury and membranous glomerulonephritis. Additionally, more than 30% of individuals with NDKD presented with chronic glomerulosclerosis, focal segmental glomerulosclerosis, IgA nephropathy and acute interstitial nephritis. The number of obsolescent glomeruli and IFTA scores showed a significant association with DN. These factors were also found to be independent predictors of the severity of DN, indirectly indicating their prognostic significance in this condition. Conducting similar studies in different settings or using a case-control study design, while adjusting for confounders such as the duration of diabetes and other associated co-morbidities, might help to generalise the findings of the current study.
¥One has been excluded as it had inadequate sample
n>61 and % >100 because multiple co-existing findings
*indicates statistically significant association at p<0.05
*indicates statistically significant difference at p<0.05
*indicates statistical significance at p<0.05