Ayurveda is a traditional Indian medicine system that has been practiced since the Vedic period [1]. Ayurvedic Pharmaceutics, also known as “Bhaishajya Kalpana,” is a discipline of pharmacy that deals with the formulation, manufacturing, and dispensing of herbal medicinal formulations [2]. The cow is considered sacred in Indian culture, and its byproducts, particularly cow’s urine, have been studied for therapeutic purposes [3]. Cow’s urine is also an important component of Panchagavya Chikitsa, often known as Cowpathy [4]. Drinking cow’s urine distillate has been used to treat numerous disorders in India for thousands of years due to its pharmacological characteristics. However, it has a short shelf life and a pungent odour, which makes it undesirable for many patients [5]; thus, modifications in its dosage forms are necessary.
A decoction is one of the fundamental preparations in Ayurvedic pharmaceutics. Various modified dosage forms of decoction are utilised to increase potency, extend shelf life, and improve palatability [6]. Ghana is one such second derivative dosage form, where water-soluble metabolites are extracted by heating to obtain a concentrated semisolid form. This semisolid mass is then dried at 50°C [7]. The antimicrobial activity of cow urine and distillate was examined using the agar well method against microorganisms such as Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis, Klebsiella pneumoniae, and Proteus vulgaris. The results indicated that fresh cow’s urine was superior to its distillate [8]. It has also been reported that phenols can kill both gram-positive and gram-negative bacteria; thus, the presence of phenols in cow’s urine may be responsible for its significant antibacterial properties. The data from the study also supported traditional practitioners’ claims regarding the antimicrobial activity of cow’s urine [9].
As scientific standards for evaluating the efficacy, stability, and safety of drugs are developed, there is an increasing need to standardise Ayurvedic formulations. The term “standardisation” refers to the employment of appropriate techniques and procedures to establish the optimal conditions for manufacturing products that meet specific requirements for purity, quality, safety, stability, and shelf life. Although many drugs are clinically successful, they are still not standardised according to the Ayurvedic Pharmacopoeia of India (API) [10]. Cow urine distillate has shown significant antibacterial activity [11]. As the availability of fresh cow’s urine has always remained a problem, especially in urban areas where access to cows may be limited, this study aims to modify it into Ghana form. However, the pharmaceutical processing and quality control methods of Gomutra Ghana remain questionable. The issues of standardisation, profiling or characterisation, and quality control remain challenges to the global acceptance of Gomutra Ghana.
Therefore, the study aimed to evaluate the pharmaceutical development and quality control assessment of Gomutra Ghana, as well as to assess its in-vitro antibacterial activity.
Materials and Methods
This was an in-vitro study. Pharmaceutical processing and analytical studies were performed in the Department of Rasashastra and Bhaishajya Kalpana at Mahatma Gandhi Ayurved College Hospital and Research Centre and Dattatraya Ayurved Pharmacy, Salod (H), Wardha, Maharashtra, India. The in-vitro (antibacterial) study was carried out in JNMC, Sawangi (M), Wardha, Maharashtra, India, from April 2022 to August 2023. Ethical approval No. MGACHRC/IEC/July 2021/358 was taken before the start of the study.
Sample collection: The region around the urethral opening in the cow was washed and cleaned properly early in the morning, then midstream cow’s urine was collected in a sterile container. Fresh cow’s urine was collected from Sarvoday Goshala Charitable Trust, Padegaon, Wardha, Maharashtra, India.
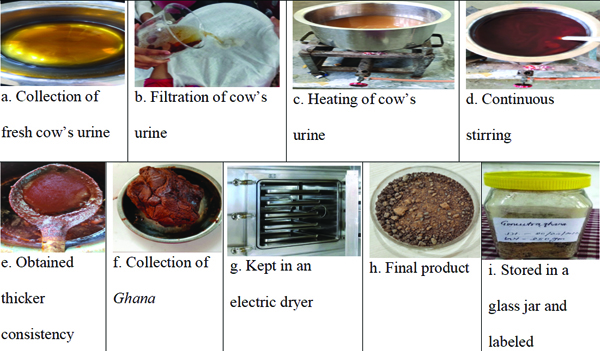
Preparation of Gomutra Ghana:Gomutra Ghana is a rasakriya (linctus) and ghanasara (semisolid dosage form) product that contains only cow’s urine. It was prepared according to the general method of rasakriya by boiling the cow’s urine until it reached a thicker consistency, as cited by Dalhana, the commentator of the Sushruta Samhita [12]. Fresh cow’s urine was collected from a Goshala (cowshed) and filtered to remove foreign matter using a clean, dry muslin cloth. The filtered urine was then placed in a stainless steel vessel and heated over a low flame at approximately 100°C. Continuous stirring was done to avoid adhesiveness and burning. The consistency was checked periodically until the desired thickness was achieved. For the drying process, it was kept uncovered in sunlight for two days and then placed in an electric dryer before being stored in a clean, sealed glass jar [Table/Fig-1]. Gomutra Ghana was prepared in three batches, with fresh cow’s urine collected from the same cow every two days.
The method of preparation of Gomutra Ghana.
Analytical study: There are no pharmacopeial standards for Gomutra Ghana; therefore, analytical research was done to establish basic parameters. The formulation and fresh cow’s urine were initially evaluated for organoleptic parameters such as colour, taste, and odour. In the analytical laboratory, samples were analysed as per API Standards [13]. The physicochemical analysis included pH [14], loss on drying at 105°C [15], total ash [16], water-soluble ash [16], acid-insoluble ash [16], water-soluble extractives [17], alcohol-soluble extractives [18], and assessment for microbial contamination [19]. Analysis was done in all batches.
High Performance Thin Layer Chromatography (HPTLC) [20]: The chromatographic analysis was conducted using the Camag Linomat IV and a Hamilton syringe (100 mL) for sample application. The development chamber employed was the Camag Twin Trough Chamber, with dimensions of 10×10 cm2. The solvent system employed was Toluene: Ethyl Acetate in a 7:3 ratio. The HPTLC analysis was performed at 254 nm. The procedure included preparation of the plates, preparation of the chamber, sample application, plate conditioning, chromatography development, detection spot, scanning, and documentation.
Antibacterial study: The in-vitro antibacterial study was carried out using the agar disc diffusion method.
Test organism: Gram-positive bacteria, Staphylococcus aureus ATCC 25923 (American Type Culture Collection) and Enterococcus faecalis ATCC 29212, were used. Gram-negative bacteria like Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 27736, and SalmonellaTyphimurium ATCC 14028 for the antimicrobial study.
Preparation of extracts: A 10 mL sample of ethanol was added to 10 mL of cow’s urine in a conical flask, which was then instantly covered and shaken. A mixture of 100 mL of ethanol and 1 g of Gomutra Ghana was shaken on a rotary shaker at 80 rpm for five hours per day over three days. The extract was subsequently filtered through Whatman No. 1 filter paper and kept in a three different 10 mL vials at 5°C for future use [21].
Preparation of culture media for the study: Aseptic technique was employed to pour 30 mL of molten sterile Mueller Hinton agar (HiMedia Lab Pvt. Ltd.) into each sterile petri dish, allowing it to solidify at room temperature. The inoculum was spread across the agar surface using a sterile swab to prepare a lawn of microorganisms [22].
Disc preparation for antimicrobial study: Whatman No. 1 filter paper was used to prepare 5 mm discs with the help of a sterile punching machine. These discs were sterilised by autoclaving at 121°C. Then the moist discs were dried in a hot air oven at 50°C. The sterile discs were kept in a small sterile bottle for further use. A Ciprofloxacin 5 μg disc was used as a standard to compare results with those from cow’s urine and Gomutra Ghana.
Antibacterial activity by disc diffusion method: A single isolated colony of Staphylococcus aureus ATCC 25923 was taken and placed into nutrient broth, which was then cultured at 37°C for 3-4 hours. The same procedure was followed for the remaining four organisms. The inoculum was evenly distributed across the entire surface of the Mueller-Hinton agar plates using a sterile cotton swab to test antibacterial sensitivity. A Ciprofloxacin 5 μg disc and the three previously prepared sterile discs were placed at a distance of 15 mm from the edge and 24 mm apart on the plate. Subsequently, 5 μL, 10 μL, and 15 μL concentrations of the prepared extracts were passed in the discs using a micropipette, onto they were positioned 15 mm from the edge and 24 mm apart. The plates were then incubated at 37°C for 24 hours. The diameter of the zone of bacterial growth inhibition around each disc was measured and recorded [21]. The susceptibility or resistance to the agent in each plate for the Ciprofloxacin 5 μg disc was assessed using the Clinical and Laboratory Standards Institute’s standardised table [23]. The zones of inhibition for cow’s urine and Gomutra Ghana were then compared to the results for Ciprofloxacin.
Statistical Analysis
The statistical analysis was done using SPSS version 27.0. The statistical test used for the analysis was ANOVA, which compared three groups: Ciprofloxacin, Cow’s urine, and Gomutra Ghana, across all three concentrations. The significance level for the antibacterial study was set at a p-value of <0.05.
Results
Gomutra ghana observations: The preparation of Gomutra Ghana using two liters of fresh cow’s urine consistently yielded around 80 grams before drying and approximately 58 grams after drying across all three batches, with a standardised drying time of 180 minutes at 50°C. The final yield showed little variation, indicating a consistent and reliable preparation process [Table/Fig-2].
The observations in Gomutra Ghana.
| S. No. | Observations in Gomutra Ghana | GG-I | GG-II | GG-III |
|---|
| 1. | Quantity of fresh cow’s urine | 2 L | 2 L | 2 L |
| 2. | Quantity of obtained Gomutra Ghana | 82.4 g | 82 g | 80 g |
| 3. | Total time for Gomutra Ghana drying in the dryer | 180 m | 180 m | 180 m |
| 4. | Temperature of dryer | 50°C | 50°C | 50 °C |
| 5. | Quantity of obtained Gomutra Ghana after drying (g) | 59.3 (2.97%) | 58 (2.90%) | 56.5 (2.83%) |
*GG-I: Gomutra ghana batch 1; GG-II: Gomutra ghana batch 2; GG-III: Gomutra ghana batch 3
Temperature observations: The temperature observations showed consistent increases across all three batches (GG-I, GG-II, GG-III) throughout the preparation of Gomutra Ghana. Starting from room temperature (~38°C) and the cow’s urine temperature (~36°C), the temperature rose gradually, reaching around 90-92°C at 30 minutes, 105-107°C at one hour, and stabilised at approximately 110-112°C from two hours onward. These observations indicate a uniform heating pattern, resulting in the characteristic brown colour and thicker consistency of the final Gomutra Ghana product after 4.5 hours [Table/Fig-3].
The observations of temperature (°C) during the preparation of Gomutra Ghana.
| Specific observations | GG-I | GG-II | GG-III |
|---|
| Room temp. | | ±38.4°C | ±39°C | ±38.2°C |
| Cow’s urine | Transparent yellow colour appearance | ±36.2°C | ±36.5°C | ±36.3°C |
| 30 mins | Light brown colour appearance, transparency was disappeared | ±90°C | ±92°C | ±80°C |
| 1 hrs | Light brown colour appearance, boiling started with vapours+ | ±105°C | ±107°C | ±100°C |
| 2 hrs | Brown colour appearance, boiling with froth + and vapors ++ | ±110°C | ±110°C | ±105°C |
| 3 hrs | Brown colour appearance, froth ++ and vapours ++ | ±110°C | ±110°C | ±107°C |
| 3.5 hrs | Brown colour appearance and thicker consistency were obtained | ±111°C | ±111°C | ±108°C |
| 4 hrs | Brown colour appearance, started sticking to the vessel | ±112°C | ±112°C | ±108°C |
| 4.5 hrs | Brown colour Ghana was prepared | ±112°C | ±112°C | ±110°C |
Results of organoleptic characteristics of Gomutra Ghana: The organoleptic characteristics were consistent across all three batches. The appearance was light brown, the taste was bitter and astringent, and no specific odour was observed.
Physicochemical parameters: The pH values of Gomutra Ghana ranged from 8.93 to 9.20, indicating an alkaline nature. The loss on drying at 105°C was around 0.5%, and the total ash content was approximately 50%. The water-soluble ash and acid-insoluble ash were consistently around 8.5% and 5.9%, respectively. The water-soluble extractives were highly consistent at around 83%, while the alcohol-soluble extractives showed slight variability, ranging from 68% to 72%. These results demonstrate the reproducibility and stability of the physicochemical properties of Gomutra Ghana [Table/Fig-4].
The results of physiochemical parameters of Gomutra Ghana.
| Sr. No. | Parameters of Gomutra Ghana | GG-I | GG-II | GG-III |
|---|
| 1. | pH | 8.93 | 9.20 | 9.04 |
| 2. | Loss on drying at 105°C | 0.52% | 0.5% | 0.49% |
| 3. | Total ash | 50.4% | 50.8% | 50.9% |
| 4. | Water soluble ash | 8.52% | 8.55% | 8.57% |
| 5. | Acid insoluble ash | 5.8% | 5.9% | 5.96% |
| 6. | Water soluble extractives | 83% | 83.4% | 83.25% |
| 7. | Alcohol soluble extractives | 69.6% | 72% | 68% |
Results of microbial load/microbial specification in Gomutra Ghana: The total viable count was a maximum of 105/g, with Klebsiella pneumoniae at a maximum of 103/g, and the total fungal count also at a maximum of 103/g. Escherichia coli showed a maximum of 105/g, while Salmonella Typhimurium, Staphylococcus aureus, and Pseudomonas aeruginosa showed no growth in all three batches.
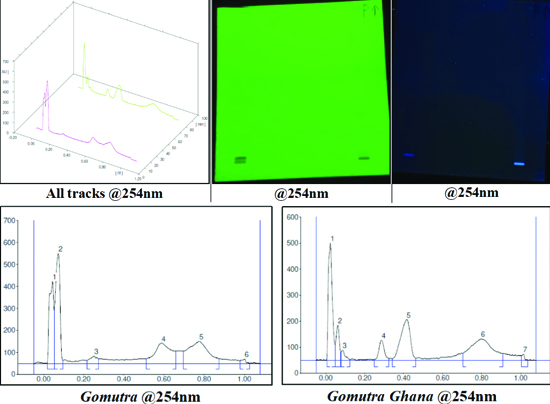
HPTLC analysis: The cow’s urine was found to have a total of six peaks with maximum Rf values (0.59, 0.78, and 1.0) and maximum areas of 5859.9, 8127.6, and 7188.7. While in Gomutra Ghana exhibited a total of seven peaks at 254 nm, with maximum Rf values (0.42, 0.80, and 1.02) and maximum areas of 5586.5, 6716.3, and 6729 [Table/Fig-5]. At 254 nm, images were obtained of both cow’s urine and Gomutra Ghana, showing the separation and identification of different compounds based on their Retention Factor (RF) values and peak areas [Table/Fig-6].
The observation and results of HPTLC of cow’s urine and Gomutra Ghana.
| Solvent system- Toluene: Ethyl acetate (7:3) |
|---|
| HPTLC of cow’s urine |
|---|
| Peak | Start Rf | Start height | Max Rf | Max height | Max % | End Rf | End height | Area | Area % |
|---|
| 1 | 0.02 | 0.6 | 0.05 | 374.3 | 33.34 | 0.05 | 241.0 | 5859.9 | 20.94 |
| 2 | 0.05 | 251.3 | 0.07 | 500.1 | 44.54 | 0.10 | 14.8 | 8127.6 | 29.04 |
| 3 | 0.22 | 14.0 | 0.25 | 33.1 | 2.95 | 0.28 | 20.6 | 932.9 | 3.33 |
| 4 | 0.51 | 22.8 | 0.59 | 93.1 | 8.29 | 0.66 | 56.6 | 5535.9 | 19.78 |
| 5 | 0.70 | 56.6 | 0.78 | 101.4 | 9.03 | 0.88 | 22.9 | 7188.7 | 25.69 |
| 6 | 0.98 | 13.7 | 1.00 | 20.7 | 1.85 | 1.03 | 1.6 | 339.3 | 1.21 |
| HPTLC of Gomutra Ghana |
| Peak | Start Rf | Start height | Max Rf | Max height | Max % | End Rf | End height | Area | Area % |
| 1 | 0.01 | 11.8 | 0.02 | 450.4 | 46.58 | 0.05 | 60.4 | 6716.3 | 29.25 |
| 2 | 0.05 | 63.7 | 0.06 | 135.4 | 14.00 | 0.08 | 29.8 | 1595.9 | 6.95 |
| 3 | 0.08 | 30.0 | 0.09 | 39.8 | 4.11 | 0.12 | 4.0 | 562.4 | 2.45 |
| 4 | 0.25 | 2.9 | 0.29 | 78.0 | 8.06 | 0.33 | 6.7 | 1544.1 | 6.72 |
| 5 | 0.34 | 5.4 | 0.42 | 156.5 | 16.19 | 0.46 | 8.0 | 5586.5 | 24.33 |
| 6 | 0.70 | 26.7 | 0.80 | 82.6 | 8.54 | 0.91 | 26.3 | 6729.1 | 29.30 |
| 7 | 0.01 | 18.1 | 1.02 | 24.3 | 2.52 | 1.04 | 1.0 | 229.9 | 1.00 |
Images of HPTLC analysis at 254 nm of Cow’s urine and Gomutra Ghana.
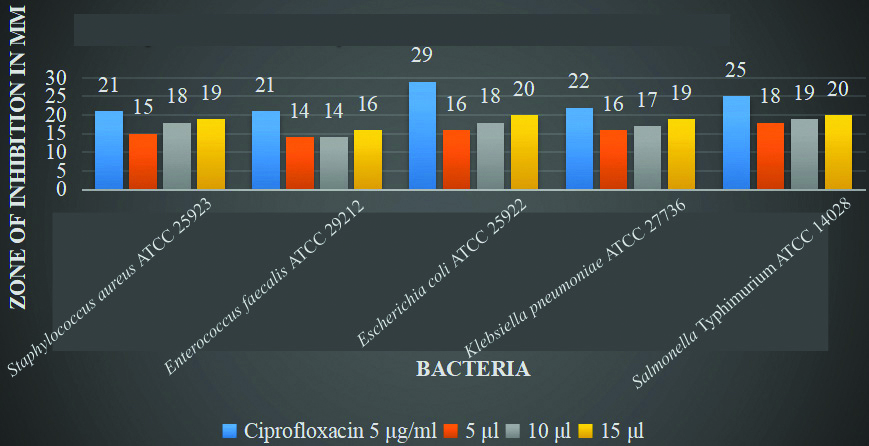
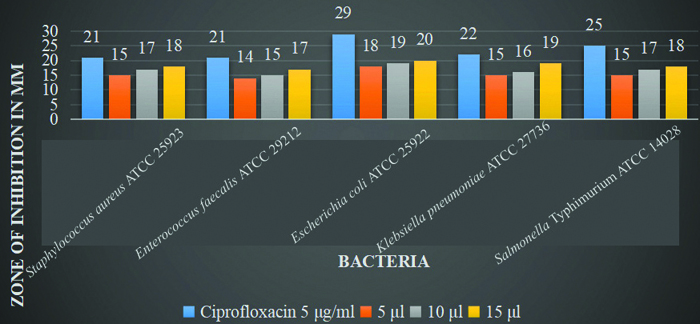
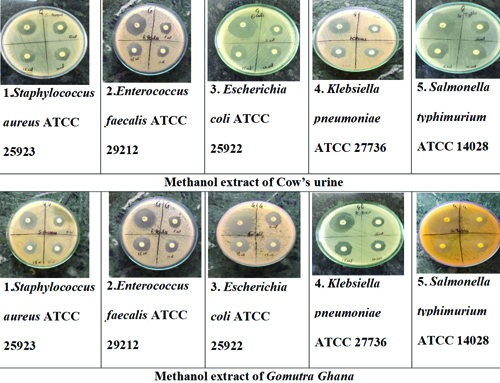
Comparison of antibacterial activity: The comparison of the methanol extract of cow’s urine and Gomutra Ghana with ciprofloxacin at different concentrations reveals notable antimicrobial activity against various microorganisms. For both extracts, the highest zone of inhibition was observed at a concentration of 15 μL. The cow’s urine extract showed the most significant inhibition against Escherichia coli ATCC 25922 (20 mm) and Salmonella Typhimurium ATCC 14028 (20 mm), with slightly lower effectiveness against other strains. Gomutra Ghana exhibited the highest inhibition against Escherichia coli ATCC 25922 (20 mm) as well, followed by Klebsiella pneumonia ATCC 27736 (19 mmm). Ciprofloxacin, the positive control, consistently demonstrated higher inhibition zones across all microorganisms, particularly against Escherichia coli (29 mm) and Salmonella Typhimurium ATCC 14028 (25 mm). Both extracts displayed dose-dependent antimicrobial activity, with increasing inhibition zones at higher concentrations [Table/Fig-7,8 and 9].
The comparison of methanol extract of cow’s urine with ciprofloxacin at different concentrations.
The comparison of the methanol extract of Gomutra Ghana with ciprofloxacin at different concentrations.
The in-vitro antibacterial activity of methanol extract of Cow’s urine and Gomutra Ghana.
A statistical comparison of the antibacterial activity among ciprofloxacin, cow’s urine, and Gomutra Ghana at three different concentrations (5 μL, 10 μL, and 15 μL) shows significant differences in the zone of inhibition (indicative of antibacterial effectiveness) among these groups at each concentration tested. Specifically, at 5 μL, the p-value of 0.0121 suggests a statistically significant difference in antibacterial activity. At 10 μL, the p-value of 0.0117 similarly indicates significant differences, and at 15 μL, the p-value of 0.0067 reaffirms significant variations in effectiveness.
The study also examines the zone of inhibition across different bacterial strains, where variability exists (5.52 to 7.79 mm) among the strains tested. These findings underscore the diverse antibacterial potentials of ciprofloxacin, cow’s urine, and Gomutra Ghana, potentially reflecting their distinct chemical compositions and mechanisms of action against bacterial pathogens [Table/Fig-10].
The statistical analysis on three groups (Ciprofloxacin, Cow’s urine and Gomutra Ghana) at 5 μL, 10 μL and 15 μL.
| ANOVA- All sample vs zone of inhibition of bacteria at 5 μL |
|---|
| Source of variation | Sum of Squares (SS) | Df | Mean Square (MS) | F | p-value |
|---|
| Various samples | 74.75 | 2 | 37.375 | 6.76749 | 0.0121 |
| Zone of inhibition at various bacteria | 60.75 | 11 | 5.522727 |
| ANOVA- All sample vs zone of inhibition of bacteria at 10 μL |
| Source of variation | SS | Df | MS | F | p-value |
| Various samples | 159.4722 | 2 | 79.73611 | 10.23351 | 0.0117 |
| Zone of inhibition at various bacteria | 46.75 | 6 | 7.791667 |
| ANOVA– All sample vs zone of inhibition of bacteria at 15 μL |
| Source of variation | SS | Df | MS | F | p-value |
| Various samples | 156.9833 | 2 | 78.49167 | 11.11855 | 0.0067 |
| Zone of inhibition at various bacteria | 49.41667 | 7 | 7.059524 |
Discussion
Gomutra Ghana, a second derivative preparation of cow’s urine, is one of the extraction process in which cow’s urine is heated until it is turned into a concentrated semisolid form [24]. This semisolid substance is then dried at 50°C, and the final product is termed as Gomutra Ghana. The study addresses the challenge of accessing fresh cow’s urine, particularly in urban areas, by transforming it into the Ghana form. Hence, in the present study, an attempt was done to study pharmaceutical development, quality control assessment, and in-vitro antibacterial activity evaluation of Gomutra Ghana.
The pharmaceutical study dealt with the preparation of a suitable dosage form of a drug material. For the quality assurance of the finished product, it is essential to evaluate and discuss the in-process conditions. Gomutra Ghana was prepared according to the Rasakriya general method in three batches. The temperature was recorded at every stage of every batch. Since mild heat was maintained, the temperature did not rise high. During the preparation of Gomutra Ghana, the transparent yellow colour of cow’s urine changed to brown. The quantity of the obtained product was much less 2.83% to 2.97%, as cow’s urine consists of 96% water [25], which evaporated during the heating process. Additionally, since Gomutra Ghana showed a hygroscopic nature due to the alkaline property of cow’s urine [26], a glass airtight jar was preferred for storage to prevent moisture content and contamination.
The pH of Gomutra Ghana indicates that it is much more alkaline than all three batches. This range of pH facilitates antacid action [27]. The loss on drying at 105°C indicates the presence of moisture content. If the moisture content exceeds the permissible limit, the formulation is more likely to be infected by fungal growth. Moreover unwanted changes can also occur due to the presence of excess moisture [28]. The moisture level in the prepared Gomutra Ghana for all batches was significantly lower and within the standard limit of 0.49% to 0.52%, suggesting that this formulation may be more stable and authentic.
The residue remaining after the incineration of the crude drug is designated as ash. Ash values help determine the quality and purity of crude drugs, especially in powdered form [29]. The total ash of a crude drug also reflects the care taken in its preparation. No significant difference was detected in the ash values of all the batches. Currently, there are no pharmacopeial standards for Ghana; hence, these values can be considered as a reference for further studies on the physicochemical parameters of Ghana.
The extractive values, namely water-soluble and alcohol-soluble, reflect the amount of active ingredient in a given amount of material when extracted with the corresponding solvents [30]. The water-soluble extractive value was higher compared to the alcohol-soluble extractive value in all batches of Gomutra Ghana.
The results of the microbial contamination analysis showed that Escherichia coli, Salmonella Typhimurium, Staphylococcus aureus, and Pseudomonas aeruginosa were not present in any of the three batches. This makes it suitable for therapeutic administration, as it was prepared under the best hygienic conditions with sterilised instruments.
For HPTLC analysis of cow’s urine and Gomutra Ghana, several solvent mixtures were experimented with, and a standard HPTLC solvent system comprising Toluene: Ethyl Acetate (7:3 v/v) was successfully developed. In Gomutra Ghana, one extra peak was found as compared to cow’s urine, indicating the formation of a new compound during the preparation process of Gomutra Ghana. The peaks from cow’s urine and Gomutra Ghana with maximum heights were 500.1 and 450.4, respectively, which were nearly the same, indicating the compounds have similar constituents, as the only ingredient in Gomutra Ghana was cow’s urine. This HPTLC pattern can be considered a reference standard for further quality control studies.
For the in-vitro antibacterial study, after experimenting with different solvents and concentrations, ethanol was selected for the extract. Antibacterial activity was assessed using the Agar disc diffusion method, which showed varying diameters of the zone of results on different bacterial strains.
When comparing the zones of inhibition, ciprofloxacin exhibited susceptibility to all the selected microorganisms and demonstrated greater antibacterial activity compared to both cow’s urine and Gomutra Ghana extracts. The highest zone of inhibition for cow’s urine extract was observed against Escherichia coli ATCC 25922 (20 mm) and SalmonellaTyphimurium ATCC 14028 (20 mm). Gomutra Ghana extract also showed the highest inhibition against Escherichia coli ATCC 25922 (20 mm). In general, the best results for the zone of inhibition were found at a concentration of 15 μL.
The results of the antibacterial study on cow’s urine were consistent with the previously studied antibacterial activity of cow’s urine (Ahuja A et al., Randhawa GK and Sharma R et al., [31,32]. In the study by Ahuja A et al., it was found that urine from various cows exhibited varying antibacterial characteristics [31]. The antibacterial effects of cow urine may vary due to its chemical components, which can change for multiple reasons. When all three samples-Ciprofloxacin, cow’s urine, and Gomutra Ghana-were compared with five types of bacteria at three different concentrations, statistically significant results were obtained across various samples and bacteria.
Cow urine possesses significant antimicrobial, germicidal, and antifungal properties due to the presence of many volatile and non volatile components such as urea, creatinine, aurum hydroxide, phenol, carboxylic acid, and calcium and manganese salts. This germicidal function is further enhanced by the presence of certain amino acids and urine peptides, which contribute to improving bacterial cell wall integrity and surface hydrophobicity [33].
The research addresses challenges related to accessibility and quality control, and shows the feasibility and assure of Gomutra Ghana as a standardised Ayurvedic formulation. Its stated antibacterial activity highlights its therapeutic potential and opens the way for further investigation into its wider pharmacological characteristics and potential applications in healthcare.
Limitation(s)
The animal and clinical evaluation of Gomutra Ghana was not performed. There may be variations in the pharmacokinetics and pharmacodynamics of the drug between animal and human bodies, which could lead to potential differences in the results of clinical studies.
Conclusion(s)
The Gomutra Ghana can be consistently prepared, yielding a stable, reproducible product with low moisture content, a stable alkaline pH, and consistent ash values. Microbial analysis confirmed the absence of contaminants, ensuring purity. HPTLC analysis identified some new compound formations, providing a reference standard for quality control. Antibacterial testing showed significant activity against various bacterial strains, particularly Escherichia coli, although it was less effective than ciprofloxacin. Therefore, Gomutra Ghana can be considered a promising Ayurvedic formulation with potential therapeutic applications. Future research should focus on broader pharmacological evaluations, studies of the mechanism of action, and clinical trials to validate these findings and optimise therapeutic use, potentially integrating Gomutra Ghana into modern healthcare practices.
*GG-I: Gomutra ghana batch 1; GG-II: Gomutra ghana batch 2; GG-III: Gomutra ghana batch 3