Endogenous endophthalmitis, a severe infection originating from the bloodstream, can lead to blindness and is particularly dangerous for immunocompromised patients, including those with diabetes, cancer, or undergoing chemotherapy. This condition differs from exogenous endophthalmitis, which arises from external sources such as postoperative complications or trauma. Hereby, the authors present a case report of a rare instance of bilateral bacterial endogenous endophthalmitis in a 45-year-old male with multiple systemic co-morbidities. The patient experienced vision loss and photophobia; further investigation revealed septicaemia linked to an in-situ haemodialysis catheter. Prompt treatment with intravenous antibiotics is crucial, and vitrectomy may also be considered. The present case is unique because, despite the infection originating in the bloodstream, the patient developed ocular symptoms first. A retrospective analysis revealed the primary source of the infection, highlighting the importance of a thorough examination.
Case Report
A 45-year-old male with a known history of diabetes mellitus, hypertension, stage 5 chronic kidney disease, and bronchial asthma presented to the Ophthalmology Outpatient Department (OPD) with complaints of vision loss and sensitivity to bright light in the right eye, as well as diminished vision in the left eye for the past 5-6 days. The patient was unable to walk on his own. He was on a regular cycle of haemodialysis (twice a week) with an in-situ catheter. On examination, his blood pressure was 160/90 mmHg. The Glasgow Coma Scale score was 13 out of 15 upon presentation. The patient appeared lethargic during the examination but was cooperative and oriented to time, place, and person.
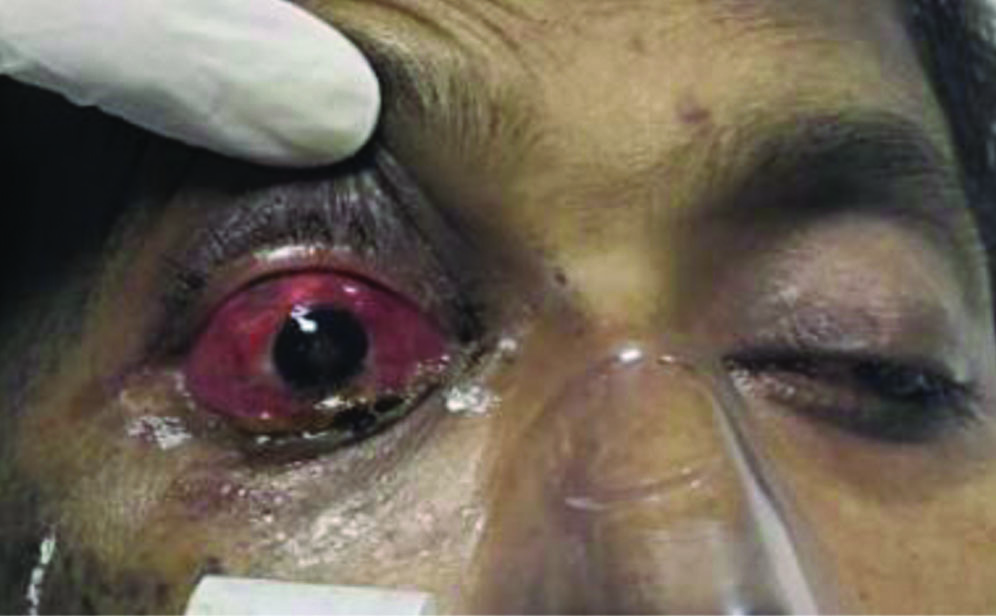
The patient presented with an absence of light perception in the right eye and a visual acuity of 20/200 in the left eye. On torch light examination, proptosis was noted in the right eye [Table/Fig-1]. A slit lamp examination revealed an exudative membrane in the anterior chamber of the right eye, covering the pupillary axis. Approximately 2 mm of hypopyon was noted inferiorly in the left eye. The pupillary reflex in the right eye was not appreciated due to the presence of the exudative membrane, while it was central, circular, and sluggishly reactive to light in the left eye. The fundus could not be visualised in the right eye, while vitritis was present in the left eye.
On torch examination of right eye, chemosis and proptosis was noted.
Based on the history, clinical signs, and symptoms, a provisional diagnosis of endogenous endophthalmitis was considered. To confirm the diagnosis, ocular ultrasonography was performed. The ultrasonography showed multiple hyperechoes and a vitreous membrane in the vitreous [Table/Fig-2]. The patient’s general condition deteriorated further, necessitating admission to the intensive care unit. Laboratory investigations indicated an active infection, with a total blood count of 17,000/mm3.
Ultrasound image of right eye, where multiple hyperechoes and vitreous membranes were noted.

Blood was sent for culture and sensitivity testing before starting any intravenous antibiotics, which indicated sensitivity to meropenem and voriconazole. The patient received an intravitreal injection of vancomycin (1 mg), voriconazole (50 μg), and ceftazidime (2.25 mg) in the left eye, and vancomycin (1 mg) in the right eye to prevent further damage on the same day. The patient was started on meropenem 500 mg twice daily, voriconazole 200 mg once daily, vancomycin 1 g once daily, and eye drops: dorzolamide twice daily, gatifloxacin plus prednisolone (Gati-P) six times a day, and homatropine twice a day.
The blood culture report was positive for methicillin-resistant Staphylococcus aureus, confirming the diagnosis of endogenous endophthalmitis. The hematogenous type was confirmed after receiving the positive culture report from the site of the in-situ haemodialysis catheter. Initially, after initiating treatment, the hypopyon in the left eye resolved. The intravitreal injection of vancomycin was repeated following the blood culture report. However, there was no visual improvement in the right eye, and vision in the left eye also deteriorated. Enucleation of the right eye was advised; however, the patient could not undergo the procedure due to systemic unfitness.
The patient was under observation for about 12 days, during which his condition deteriorated systematically, and panophthalmitis developed in the right eye despite treatment. Despite extensive efforts to manage the patient with available treatments, he could not be saved.
Discussion
The present case involved a patient with complex systemic conditions who presented to the Ophthalmology OPD with ocular symptoms. Further evaluation traced the source of the infection, revealing endogenous endophthalmitis via a hematogenous route, originating from an in-situ haemodialysis catheter. This was confirmed by a positive culture report obtained from the site of the in-situ haemodialysis catheter.
The endogenous form of endophthalmitis is relatively rare, constituting only 2-8% of all endophthalmitis cases [1]. Moreover, among those with endogenous endophthalmitis, around 12% experience the condition bilaterally, meaning both eyes are affected [2]. However, there has been an increase in the number of cases of endogenous endophthalmitis as the population of chronically ill patients grows and the use of invasive procedures rises. This condition occurs when the posterior segment of the eye is involved in the presence of an infective source in the body. While it can affect both eyes, it mostly affects one eye, particularly the right eye, due to its direct blood flow from the heart to the right carotid artery [3]. Individuals with immunodeficient conditions are at a higher risk of developing it, especially those with diabetes mellitus, where the growth of microorganisms is more pronounced due to the high availability of blood glucose [4]. Symptoms of this condition include redness, photophobia (increased sensitivity to bright light), loss of vision, diminished vision, proptosis, and hypopyon. Investigations include blood culture, intraocular culture, and ocular ultrasonography. Treatment options include intravenous antibiotics (empiric broad-spectrum antibiotic therapy) and vitrectomy [4].
The organisms causing endogenous endophthalmitis are primarily either bacteria or fungi. The most frequently identified causative bacteria are Staphylococcus aureus and Streptococcus pneumoniae. In East Asia, however, Klebsiella pneumoniae is predominantly responsible. Among fungal organisms, Candida albicans is the most common yeast, while Aspergillus is the most common mould. Staphylococcus aureus is a versatile Gram-positive bacterium that can cause various clinical manifestations. Managing it can sometimes be challenging, especially as more cases of Methicillin-resistant Staphylococcus Aureus (MRSA) emerge [5,6].
In a study conducted on 34 affected eyes in 27 patients, the mean age was found to be 63 years, with a range of 30 to 85 years [7]. Risk factors for developing endogenous endophthalmitis include diabetes mellitus, long-term corticosteroid use, immunosuppression, a history of recent hospitalisation, ventilator-associated pneumonia, malignancies such as lymphoma and leukaemia, colon adenocarcinoma, urinary tract infections, dental infections, scleral perforation, vertebral osteomyelitis, liver abscess, asplenia, hypogammaglobulinemia, cardiac or renal transplantation, acquired immune deficiency syndrome, chronic alcoholism, intravenous catheters, and drug abuse [8-11].
The incidence of endogenous endophthalmitis ranges from 2% to 8%, making it a rare pathology. Symptoms that may raise suspicion of endogenous endophthalmitis include pain, photophobia, diminished vision, and redness. Clinical signs include periorbital oedema, conjunctival congestion, chemosis, cells or flare in the anterior chamber, hypopyon, abnormal pupillary reactions, absence of the red reflex, vitreous haze, and the presence of exudates and membranes. To confirm the diagnosis, author relied on several diagnostic modalities: B-scan ultrasonography, which shows heterogeneous opacities and echoes in the vitreous cavity when the posterior segment is involved, and optical coherence tomography, which helps to identify the location of exudates within the retinal layers. An anterior chamber or vitreous tap can be performed to obtain a sample of 0.1-0.2 mL or 1-2 mL, which is then sent for culture and sensitivity testing. This helps identify the exact causative organism, allowing for specific management of the case [12].
A vigorous treatment approach is necessary, as this condition has a very poor prognosis even after treatment. Management includes systemic antibacterials, as well as topical and intravitreal agents. The standard doses of intravitreal antibacterial agents are as follows: vancomycin 1 mg/0.1 mL, ceftazidime 2.25 mg/0.1 mL, or amikacin 0.4 mg/0.1 mL [13]. Surgical options include pars plana vitrectomy [14]. Chronic kidney disease is one of the co-morbidities associated with the development of endogenous endophthalmitis. The pathogenesis is thought to be related to the in-situ haemodialysis catheter, which can be a primary source of infection [14]. The prognosis depends on various factors, including the patient’s associated co-morbidities, overall condition at the time of presentation, extent of the pathology, and the causative organism.
Conclusion(s)
The final diagnosis of endogenous endophthalmitis following haemodialysis was made when the patient presented to the Ophthalmology OPD with vague ocular complaints. Given the wide range of possible differential diagnosis based on the initial symptoms, a thorough assessment that considered the patient’s existing co-morbidities and a detailed examination were essential. This comprehensive approach led to the identification of both the diagnosis and the primary cause of the condition.
[1]. Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS, Endogenous bacterial endophthalmitis. Report of a ten-year retrospective studyOphthalmology 1994 101:832-38.Available from: https://pubmed.ncbi.nlm.nih.gov/8190467/10.1016/S0161-6420(13)31255-X8190467 [Google Scholar] [CrossRef] [PubMed]
[2]. Jackson TL, Eykyn SJ, Graham EM, Stanford MR, Endogenous bacterial endophthalmitis: A 17-year prospective series and review of 267 reported casesSurv Ophthalmol 2003 48:403-23.10.1016/S0039-6257(03)00054-712850229 [Google Scholar] [CrossRef] [PubMed]
[3]. Greenwald MJ, Wohl LG, Sell CH, Metastatic bacterial endophthalmitis: A contemporary reappraisalSurv Ophthalmol 1986 31(2):81-101.10.1016/0039-6257(86)90076-73541265 [Google Scholar] [CrossRef] [PubMed]
[4]. El-Mollayess GM, Saadeh JS, Salti HI, Exogenous endophthalmitis in diabetic patients: A systemic reviewISRN Ophthalmol 2012 2012:456209PMCID: PMC391259910.5402/2012/45620924555128 [Google Scholar] [CrossRef] [PubMed]
[5]. Ho V, Ho LY, Ranchod TM, Drenser KA, Williams GA, Garretson BR, Endogenous methicillin-resistant Staphylococcus aureus endophthalmitisRetina 2011 31(3):596-601.10.1097/IAE.0b013e3181ecccf021343874 [Google Scholar] [CrossRef] [PubMed]
[6]. Taylor TA, Unakal CG, Staphylococcus aureus Infection. [Updated 2023 Jul 17]In: StatPearls [Internet] 2024 Jan Treasure Island (FL)StatPearls PublishingAvailable from: https://www.ncbi.nlm.nih.gov/books/NBK441868/ [Google Scholar]
[7]. Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM, Endogenous endophthalmitis: An 18-year review of culture-positive cases at a tertiary care centerMedicine (Baltimore) 2003 82(2):97-105.10.1097/00005792-200303000-0000412640186 [Google Scholar] [CrossRef] [PubMed]
[8]. Durand ML, EndophthalmitisClin Microbiol Infect 2013 19(3):227-34.10.1111/1469-0691.1211823438028 [Google Scholar] [CrossRef] [PubMed]
[9]. Lamaris GA, Esmaeli B, Chamilos G, Desai A, Chemaly RF, Raad II, Fungal endophthalmitis in a tertiary care cancer center: A review of 23 casesEur J Clin Microbiol Infect Dis 2008 27(5):343-47.10.1007/s10096-007-0443-918183439 [Google Scholar] [CrossRef] [PubMed]
[10]. Keyashian K, Malani PN, Endophthalmitis associated with intravenous drug useSouth Med J 2007 100(12):1219-20.10.1097/SMJ.0b013e318158119118090965 [Google Scholar] [CrossRef] [PubMed]
[11]. Cheng HH, Ding Y, Wu M, Tang CC, Zhang RJ, Lin XF, Endogenous aspergillus endophthalmitis after kidney transplantationInt J Ophthalmol 2011 4(5):567-71. [Google Scholar]
[12]. de Lima LM, Cecchetti SA, Cecchetti DF, Arroyo D, Romão EA, Dantas M, Endophthalmitis: A rare but devastating metastatic bacterial complication of hemodialysis catheter-related sepsisRen Fail 2012 34(1):119-22.10.3109/0886022X.2011.62355722017567 [Google Scholar] [CrossRef] [PubMed]
[13]. Niyadurupola N, Astbury N, Postoperative endophthalmitisCommunity Eye Health 2015 28(90):32-33.PMCID: PMC467526226692647 [Google Scholar] [PubMed]
[14]. Gurnani B, Kaur K, Endogenous Endophthalmitis. [Updated 2023 Jun 11]In: StatPearls [Internet] 2024 Jan Treasure Island (FL)StatPearls PublishingAvailable from: https://www.ncbi.nlm.nih.gov/books/NBK576391/ [Google Scholar]