Periodontal diseases are a group of prevalent oral health conditions that affect the supporting structures of teeth, including the gums, periodontal ligament, and alveolar bone [1]. These diseases are primarily caused by the accumulation of dental plaque, a complex biofilm comprising bacteria and their by-products, which leads to inflammation, tissue destruction, and potential tooth loss if left untreated. Traditional methods of periodontal therapy involve mechanical scaling and root planing to remove bacterial deposits and promote tissue healing [2]. While effective, these approaches may have limitations in sites with difficult access and may not fully address the rising concerns of antibiotic resistance [3].
In recent years, PDT has emerged as a promising non invasive treatment approach for various infections, including periodontal diseases caused by dental plaque [4]. PDT utilises low-power lasers with specific wavelengths in combination with PSs drugs to selectively target and destroy microorganisms. The activation of photosensitising compounds by light initiates a photochemical reaction, leading to the production of cytotoxic reactive oxygen species, particularly singlet oxygen, which effectively kills bacteria [5].
The simplicity and high efficacy of bacterial killing with PDT have led to its extensive use as an antimicrobial therapy in various medical fields [6]. In periodontology, PDT offers a potential alternative to traditional mechanical methods, with the advantage of addressing concerns about bacterial resistance and providing an adjunct to non-surgical periodontal therapy [7].
The findings from this systematic review will contribute to a better understanding of the potential of PDT in periodontology, its limitations, and areas for further research. With the rising concerns of antibiotic resistance and the need for innovative and effective treatments, exploring PDT’s role in periodontal disease management holds promise for revolutionising the way we approach the treatment of periodontal diseases and combating dental plaque-associated infections.
The present systematic review aimed to comprehensively assess the existing literature on the application of PDT in periodontology. By exploring the current evidence, authors aimed to evaluate the scope of PDT in periodontal disease management, its impact on clinical outcomes, factors affecting its efficacy, and potential safety considerations. Furthermore, authors will identify gaps in the literature and provide insights into future perspectives and recommendations for integrating PDT into clinical practice.
Materials and Methods
A comprehensive electronic search was conducted in major medical databases, including PubMed, Embase, and the Cochrane Library, to identify relevant studies published upto the date of this review. The following keywords and Medical Subject Headings (MeSH) terms were used: “Chronic Periodontitis,” “Scaling and Root Planning,” “Non Surgical Periodontal Therapy,” “Subgingival,” and “PDT,” as shown in [Table/Fig-1]. After conducting a thorough analysis, duplicates were eliminated based on relevant databases, titles, authors, publication years, and abstracts. For present review article, a meticulous manual screening process was carried out to ensure the removal of duplicates.
Showing the Patient/Population Intervention Comparison and Outcomes (PICOS) parameters.
| Variables | Population | Intervention | Comparison | Outcome | Study model |
|---|
| Key concepts | Chronic periodontitis | Photodynamic Therapy (PDT) | Test: SRP+PDT control: SRP | CAL/PPD/PI/GI | Clinical trials |
| MeSH terms | Chronic periodontitis | Photodynamic Therapy (PDT) Indocyanine Green (ICG) laser | Scaling and root planning | - | - |
| Free text terms | Adult periodontitis | SRP+aPDT | SRP+aPDT showed significantly greater reduction and gain compared to SRP alone at all time points, with no observed adverse affects of aPDT | Clinical Attachment Level (CAL)Probing Pocket Depth | - |
SRP: Scaling and root planning; aPDT: Anti-microbial photodynamic therapy; PDT: Photodynamic therapy; CAL: Clinical attachment level; PPD: Probing pocket depth; PI: Plaque index; GI: Gingival index; MeSH: Medical subject headings
Inclusion criteria: Randomised Controlled Trails (RCTs), case-control studies, and cohort studies involving human subjects (both parallel and split-mouth designs) from 2007 to 2023 were included. Studies in which either PSs, ICG, or antimicrobial PDT (aPDT) was used for subgingival irrigation or subgingival application as an adjunct to scaling and root planning in chronic periodontitis patients were considered. Studies reporting outcomes such as probing pocket depth, Clinical Attachment Level (CAL), Plaque Index (PI), Gingival Index (GI), and other correlated outcomes were included.
Exclusion criteria: Animal studies, in-vitro studies, and studies in which patients were under systemic medication that may affect clinical outcomes were excluded. Additionally, studies that did not have proper follow-up and articles published in languages other than English were also excluded. Studies in which interventions were conducted using other antimicrobials were not considered.
In present review article, the included studies were selected from electronic databases covering the past 16 years, specifically from 2007 to 2023. After a complete analysis, duplicate entries were eliminated based on the titles and abstracts of the studies [Table/Fig-2].
Preferred Reporting Items for Systemic Reviews and Meta Analyses (PRISMA) showing flow diagram for new systematic reviews which included searches of databases.
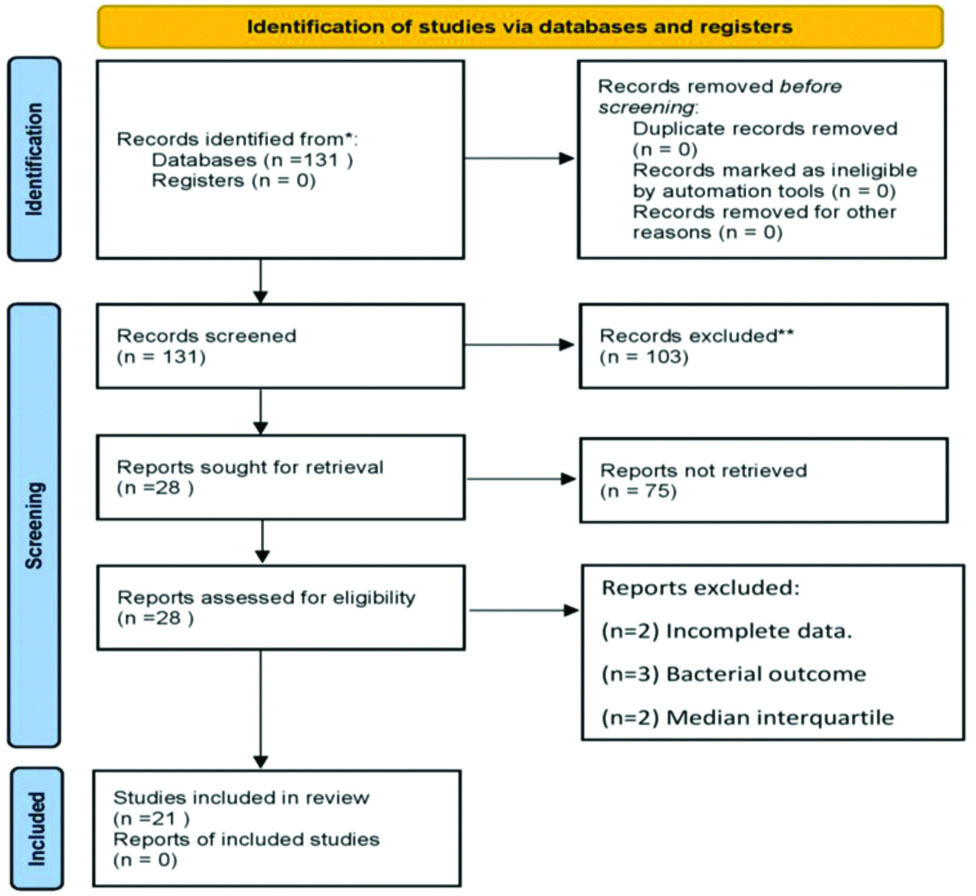
Study Procedure
Data assessment: The risk of bias for RCTs was assessed using the Cochrane Collaboration tool and performed with RevMan 2 software [8]. Risk of bias was evaluated by two independent reviewers for the RCTs included in the review, and discrepancies were resolved through discussion and consultation with a third reviewer. The domains for risk assessment were graded as high, unclear, or low risk based on selection bias, random sequence generation, allocation concealment, selective reporting, other bias, blinding of participants, blinding of outcome assessment, incomplete outcome data, and overall risk of bias. A study was assessed to have a low overall risk only if, all domains were found to have low risk; it was assessed to have a high overall risk if, one or more of the six domains were found to be at high-risk. An unclear risk assessment was assigned to studies when one or more domains were uncertain, provided none were at high-risk.
Results
Risk of bias: RevMan software version 5.4 was used to analyse the risk of bias in the present study. Authors evaluated individual studies for various domains, including selection bias (creation of a random sequence), performance bias (blinding of cases and staff), attrition bias (incomplete results data), selective reporting (reporting bias), and other biases. Each study was classified as having low risk (+), high-risk (-), or unclear risk (?), as depicted in [Table/Fig-3] [9-29]. The present review of PDT in periodontology consolidated 43 articles into 21 relevant RCTs, as highlighted in [Table/Fig-3], providing a comprehensive summary of findings. Among these studies, the most concerning issues were the inadequacy or absence of randomisation, which resulted in a high-risk for 1 out of 168 trials (0.59%). In contrast, low risk was noted in 139 out of 168 trials (82.74%), and unclear risk was noted in 28 out of 168 trials (16.67%), as represented in [Table/Fig-4].
Randomised Controlled Trials (RCT) on PDT [9-29].
| Author and year | Place of the study | Study design | Aim | Treatment protocol/follow-up | Dye used | Clinical parameters assessed | Results |
|---|
| Andersen R et al., 2007 [9] | USA | RCT | To compare the effectiveness of a photo disinfection process to that of scaling and root planing for non surgical periodontal treatment | Group 1-PDGroup 2-SRP aloneGroup 3-SRP+PD6 and 12 weeks | Methylene blue | BOP1PPD2CAL3 | Significant results at 6 and 12 weeks in terms of BOP-71% and 73% in Group 1CAL-0.92±0.62 and 0.86±0.61 in Group 2PPD-1.16+0.39 and 1.11±0.53 in Group 2 |
| Christodoulide N et al., 2008 [10] | Netherlands | Randomised control clinical trial | To evaluate the clinical and microbiological adjunctive uses of aPDT for non surgical periodontal treatment in chronic periodontitis | Group 1-SRPGroup 2-aPDT+SRP3 and 6 months | Methylene blue | FMPS4FMBS5BOPPPD | No significant differences were observed between the groups in terms of clinical parameters |
| Lulic M et al., 2009 [11] | Switzerland | Randomised control clinical trial | To evaluate possible added benefits of repeated adjunctive aPDT to conventional treatment of residual pockets in patients enrolled in periodontal maintenance | Group 1-LaserGroup 2-aPDT3, 6 and 12 months | Methylene blue | PPDCALBOP | Significant results observed in test group;PPD reduction-6.08±1.19 to 5.41±1.09 at 6 monthsCAL gain-6.70±2.17 to 6.18±2.26 at 6 monthsBOP% decreased-97% to 67% at 6 months |
| Rühling A et al., 2010 [12] | Germany | Prospective, randomised, controlled, single-blind clinical study | Evaluate whether aPDT can reduce Probing Depth (PD) in persistent periodontal pockets, change the microbial composition, and decrease the total load of subgingival bacteria more than conventional mechanical debridement | Group 1-USTGroup 2-aPDT3 months | 5% tolonium chloride (toluidine blue) | PDRAL6BOP | No significant differences were observed between the groups |
| Theodoro LH et al., 2012 [13] | Brazil | - | Evaluate the long-term clinical and microbiological effects of aPDT associated with non surgical periodontal treatment | Group 1-SRP groupGroup 2-SRP+aPDT60, 80, 90 days | Toluidine blue | PI7BOPPD8GR9CAL | No significant differences were observed between the groups |
| Mongardini C et al., 2012 [14] | Italy | Single-blinded, split-mouth design, randomised parallel clinical trial | To study the potential adjunctive effect of microbiological/clinical photodynamic protocol using an Light Emitting Diode (LED) lamp (red spectrum) and to compare it to SRP | Group 1-SRPGroup 2-toluidine blue+LED lamp7 days | Toluidine blue | PPDBOP | Significant results in test group: BOP was reduced by 71% than control Group-27% |
| Balata ML et al., 2013 [15] | Brazil | Randomised, blinded, controlled clinical trial | To evaluate an aPDT protocol as an adjunct to ultrasonic debridement in patients with Severe Chronic Periodontitis (SCP) | Group 1-SRPGroup 2-aPDT1, 3 and 6 months | Methylene blue | PIGI10BOPPPDCAL | No significant differences were observed between the groups |
| Macedo GDO et al., 2013 [16] | Brazil | Randomised, controlled clinical trial | To evaluate the aPDT combined with non surgical periodontal and doxycycline on clinical and metabolic effects in patients that show type 2 diabetes mellitus | Group 1-SRPGroup 2-SRP+aPDT, both the group in combination were givendoxycycline (100 mg/day, for 2 weeks) 3 months | Phenothiazine chloride | PPDCALBOP | No significant differences were observed between the groups in terms of clinical parameters. However, the test group exhibited greater differences in HbA1c between baseline and 3 months |
| Betsy J et al., 2014 [17] | India | Single-centered randomised and controlled clinical trial | To evaluate the potential of antimicrobial Photodynamic Therapy (aPDT) as an adjunct to Scaling and Root Planing (SRP) in the treatment of chronic periodontitis | Group 1-SRPGroup 2-SRP+aPDT1, 3 and 6 months | Methylene blue | PPDCALGIGBI11PI | Significant results in test groupPPD reduction-5.7 (5.0-6.0;1.0) to 3.0 (2.0-6.0;1.0)CAL gain-6.5 to 4.0PI-2.0 to 1.0GI-2.0 to 1.0GBI-100 to 25 |
| Carvalho VF et al., 2015 [18] | Brazil | Randomised controlled parallel-group clinical trial | Evaluate the clinical and microbiological effects of aPDT in the treatment of residual pockets of patients with chronic periodontitis subjected to supportive therapy | Group 1-saline solutionGroup 2-PS+light3, 6 and 12 months | Methylene blue 0.01% | BOPPIPDCAL | No significant differences were observed between the groups |
| Moreira AL et al., 2015 [19] | Brazil | Split-mouth double-masked randomised controlled clinical trial | To study the efficiency of multiple sessions of aPDT in combination with SRP versus SRP in patients that show aggressive periodontitis | Group 1-SRPGroup 2-SRP+aPDT30 and 90 days | Phenothiazine chloride | BOPPDCALGRPI | Significant results in test groupPPD-5.32±0.34 to 2.91±0.45CAL-5.38±0.93 to 3.85±0.91GR-0.06±0.27 to 0.93±1.04BOP-144 (60.00) to 22 (13.75) |
| Pulikkoti SJ et al., 2016 [20] | Malaysia | Randomised split-mouth controlled clinical trial | To evaluate the efficacy of aPDT in reducing Aggregatibacter actinomycetemcomitans (Aa) in periodontitis patients | Group 1-NSPTGroup 2-NSPT+PDT7 days, 1 month and 3 months | Methylene blue | PDCALBOP(PS%) | Significant results in test group:BOP decreased from 56.84±26.12 to 12.44±20.4 |
| Shingnapurkar SH et al., 2016 [21] | India | Comparative split-mouth randomised clinical trial | To assess the effect of adjunctive Photodynamic Therapy (PDT) {using 810 nm diode laser and Indocyanine Green (ICG) as Photosensitisers (PSs)} in chronic periodontitis | Group 1-SRPGroup 2-SRP+PDT1 and 3 months | Indocyanine Green (ICG) | PIGIPPDRAL | Significant results in test group:PI-1.07+0.28 to 0.26+0.34GI-1.72±0.56 to 0.18±0.27PPD reduction-5.13±0.34 to 2.23±0.67RAL-9.13±1.88 to 6.60±1.4 |
| Raut CP et al., 2018 [22] | India | Randomised, single-blind, controlled clinical trial | To compare and evaluate the effects of photothermal therapy using ICG in the treatment of chronic periodontitis with Scaling And Root Planing (SRP) | Group 1-SRPGroup 2-(SRP+photothermal therapy)6 months | Indocyanine Green (ICG) | PIBOPPPDCAL | Significant results in test group:BOP reduced from 100% to 10%PPD reduction-6.04±0.82 to 3.53±0.58CAL gain-5.80±0.70 to 4.12±0.78 |
| Cadore UB et al., 2019 [23] | Brazil | Double-blind, randomised, controlled, and split-mouth clinical trial | To evaluate the clinical effects and the subgingival microbiota after multiple sessions of aPDT associated with surgical treatment of Severe Chronic Periodontitis (SCP) | Group 1-multiple sessions of aPDT and surgical periodontal treatment (ST)Group 2-ST only60, 150 days | Not mentioned | PDCALGRBOPPI | Significant results in test group:PPD reduction-6.43±0.21 to 3.31±0.18CAL gain-7.00±0.27 to 5.03±0.36 |
| Borekci T et al., 2019 [24] | Turkey | Prospective controlled clinical study | To evaluate the microbiological and clinical effects of aPDT as an adjunctive tool to the non surgical periodontal protocol in patients that show aggressive periodontitis (agp) | Group 1-NSPTGroup 2-NSPT+PDT63 days | Toulidine blue | PISBI12PPDRALGR | Significant result in test group:SBI reduced from 3.51±0.73 to 0.96±3.41 |
| Niazi FH et al., 2020 [25] | Saudi Arabia | Double-blind, RCT | To evaluate clinical periodontal and microbiological parameters after the treatment with adjunctive antimicrobial aPDT among Human Immunodeficiency Virus (HIV)-seropositive and seronegative patients with necrotising ulcerative periodontitis | Group 1-seropositive patientsGroup 2-Healthy3 and 6 months | Methylene blue | FMPI13FMBOP14PDCAL | Significant results in test group:FMBOP%-69.7±22.5 to 14.8±9.2bPD-5.0±1.4 to 3.3±0.9b |
| Joshi K et al., 2020 [26] | India | Single centre split mouth randomised controlled clinical study | To assess the clinical efficacy of ICG, and PSs with better tissue absorption and low toxicity, as an aPDT adjuvant to Scaling and Root Planing (SRP) | Group 1-SRPGroup 2-SRP+PDT3 months | Indocyanine Green (ICG) | PImSBI15PPDCAL | Significant results in test group:PPD reduction-5.56±0.55 to 3.20±0.54CAL gain-5.68±0.61 to 3.34±0.62 |
| Patyna M et al., 2021 [27] | Germany | Single-blinded, randomised, controlled clinical pilot study | To evaluate the microbiological and clinical effects of aPDT procedure alone or in combination with probiotics as an adjunct to non surgical periodontal treatment | Group 1-SDGroup 2-SD+LADGroup 3-SD+LAD+Probiotic3 and 6 months | Toluidine blue | PPD CALBOPGIs16PCR17 | Significant result in Group 3:BOP-34.00 (±25.30) to 4.88 (±6.72)GIs-29.09 (±25.12) to 3.18 (±5.33) |
| Alshibani N et al., 2022 [28] | Saudi Arabia | Parallel-armed RCT | To assess the effect of Non surgical Periodontal Therapy (NSPT) with adjunct Photodynamic Treatment (PDT) for the management of periodontal inflammation in young Electronic cigarette (E-cig) users | Group 1-NSPT+PDTGroup 2-NSPT alone6 months | Not mentioned | PIBICALPD | No significant differences were observed between the groups |
| Skalerič E et al., 2023 [29] | Slovenia | RCT | To compare the long-term results of antimicrobial PDT (aPDT) and antibiotic therapy as an adjunct to conventional non-surgical therapy in patients with aggressive periodontitis | Group 1-NSPT+aPDTGroup 2-NSPT+antibiotics (amoxicillin 500 mg and metronidazole 400 mg, 7 days)6, 9 and 12 months | Methylene blue | PDCALBOP | No significant differences were observed between the groups |
1BOP: Bleeding on probing; 2PPD: Pocket probing depth; 3CAL: Clinical attachment loss; 4FMPS: Full mouth plaque score; 5FMBS: Full mouth bleeding score; 6RAL: Relative attachment loss; 7PI: Plaque index; 8PD: Probing depth; 9GR: Gingival recession; 10GI: Gingival index; 11GBI: Gingival bleeding index; 12SBI: Sulcular bleeding index; 13FMPI- full mouth plaque index; 14FMBOP: Full mouth bleeding on probing; 15mSBI-modified sulcular bleeding index; 16GI: Gingival index simplified; 17PCR: Plaque control record
Assessment of study quality [9-29].
| Author and year | Random sequence generation | Allocation concealment | Selective reporting | Other bias | Blinding of participants | Blinding of outcome assessment | Incomplete outcome data | Overall risk of bias |
|---|
| Andersen R et al., 2007 [9] | Unclear | Low | Low | Low | Low | Unclear | Low | Unclear |
| Christodoulide N et al., 2008 [10] | Low | Unclear | Low | Low | Low | Low | Low | Low |
| Lulic M et al., 2009 [11] | Low | Low | Low | Low | Low | Low | Low | Low |
| Rühling A et al., 2010 [12] | Low | Unclear | Low | Low | Low | Low | Low | Low |
| Theodoro LH et al., 2012 [13] | Unclear | Unclear | Low | Low | Unclear | Unclear | Low | High |
| Mongardini C et al., 2012 [14] | Low | Unclear | Low | Low | Low | Low | Low | Low |
| Balata ML et al., 2013 [15] | Low | Low | Low | Low | Low | Low | Low | Low |
| Macedo GDO et al., 2013 [16] | Low | Unclear | Low | Low | Low | Low | Unclear | Medium |
| Betsy J et al., 2014 [17] | Low | Low | Low | Low | Low | Low | Low | Low |
| Carvalho VF et al., 2015 [18] | Low | Low | Low | Low | Low | Low | Low | Low |
| Moreira AL et al., 2015 [19] | Low | Low | Low | Low | Low | Low | Low | Low |
| Pulikkoti SJ et al., 2016 [20] | Low | Unclear | Low | Low | Low | Unclear | Low | Medium |
| Shingnapurkar SH et al., 2016 [21] | Low | Unclear | Low | Low | Unclear | Unclear | Low | Medium |
| Raut CP et al., 2018 [22] | Low | Unclear | Low | Low | Low | Low | Low | Low |
| Cadore UB et al., 2019 [23] | Low | Low | Low | Low | Low | Low | Low | Low |
| Borekci T et al., 2019 [24] | Low | Unclear | Low | Low | Low | Low | Low | Low |
| Niazi FH et al., 2020 [25] | Low | Low | Low | Low | Low | Low | Low | Low |
| Joshi K et al., 2020 [26] | Low | Unclear | Low | Low | Low | Unclear | Low | Unclear |
| Patyna M et al., 2021 [27] | Low | Low | Low | Low | Low | Low | Low | Low |
| Alshibani N et al., 2022 [28] | Low | Low | Low | Low | Low | Low | Low | Low |
| Skalerič E et al., 2023 [29] | Low | Low | Low | Low | Unclear | Unclear | Low | Medium |
The assessment of study quality within the pool of 21 included studies was conducted using the Cochrane risk of bias tool, as detailed in [Table/Fig-4] [9-29]. Among the 21 studies, one study showed a high-risk of bias [13], and six of them were found to exhibit an overall unclear risk of bias [9,16,20,21,26,29]. Despite this unclear risk, the quality of these studies was deemed acceptable, suggesting that while they had some methodological limitations, they still provided valuable contributions to the research area.
In contrast, the majority of the studies, specifically 17 out of the 21, demonstrated an overall low risk of bias [9-29]. These studies were recognised for their robust methodology and study design, resulting in a classification of good quality. Their lower risk of bias underscores the reliability and trustworthiness of their findings within the context of the systematic review.
Study characteristics: In the present review, approximately 21 studies were included, which primarily focused on evaluating aPDT alongside traditional methods such as ultrasonic debridement and Scaling and Root Planing (SRP). Thirteen studies specifically compared these approaches, with five studies showing significant improvements in outcomes like Bleeding on Probing (BOP), Probing Pocket Depth (PPD), and Clinical Attachment Level (CAL) gain [17,19,21,22,26]. Two studies reported enhancements in BOP only [14,20]. However, five studies did not find notable differences between aPDT combined with conventional treatments and conventional treatments alone [10,13,15,16,28].
The aPDT protocols in the trials used various PSs like phenothiazine chlorine, methylene blue, toluidine blue, and Indocyanine Green (ICG). These were incubated for upto five minutes. Different light strategies were employed depending on the PS, with ICG treated at 810 nm and others at 628 to 680 nm. Light power ranged from 2 to 100 mW/cm2, with energy levels between 20 and 320 J/cm2 [10-15]. Moreover, the integration of ICG as a PS was consistently combined with conventional treatments in the studies, showing clinical improvements. Follow-up durations ranged from seven days to 12 months, allowing for the assessment of both short-term and long-term effects.
Discussion
The systematic review on PDT in periodontology has provided valuable insights into the potential of this innovative treatment approach for managing periodontal diseases caused by dental plaque. This discussion will delve into the key findings, implications, and future perspectives identified in the review, shedding light on the current evidence and its significance in the field of periodontal therapy.
Effectiveness of PDT in periodontal disease management: The review demonstrated that PDT, when used as an adjunct to conventional periodontal treatments, can lead to significant clinical improvements. The ability of PDT to target and kill microorganisms through the generation of cytotoxic reactive oxygen species, particularly singlet oxygen, provides a promising alternative to traditional mechanical approaches for eliminating bacterial deposits [30,31]. These findings suggest that PDT has the potential to enhance the outcomes of non-surgical periodontal therapies and address the limitations of conventional methods in sites with difficult access.
Effectiveness of PDT in various test groups: Similarly, another study introduced a test group infused with probiotics in conjunction with PDT and SRP. The outcomes reported by Patyna M et al., favoured this combination, resulting in significant clinical improvements and a microbiological achievement marked by a substantial reduction of specific pathogens [27].
The review also included two studies that explored the implications of repeated applications of PDT in supportive periodontal therapy. The outcomes of these studies underscored the potential for multiple applications of adjunctive PDT to yield improved clinical results in residual pockets among maintenance patients [11,12].
Furthermore, a comparison between PDT and antibiotics in the context of scaling and root planing revealed comparable long-term improvements in periodontal parameters for both interventions. Another investigation examined the adjunctive effect of PDT in surgical periodontal therapy, showing significant reductions in probing depth and gains in clinical attachment level for the PDT group. Notably, changes in the subgingival microbiota were consistent across both groups; however, the PDT group exhibited a higher concentration of bacteria associated with periodontal disease at the conclusion of the study [29].
Andersen R et al., and Christodoulides N et al., employed more complex methodologies in their investigations, each characterised by three distinct study arms. One study introduced a supplementary experimental group exclusively undergoing PDT, with three study arms designed to comprehensively investigate the effects of different interventions. Each arm represented a unique aspect, enhancing internal validity and providing insights into the effectiveness of the interventions. In a prior investigation, patients underwent SRP before being randomised into either a no further treatment arm or an adjunctive PDT arm [9,10].
Variability in study outcomes: While the majority of studies demonstrated favourable outcomes, it is important to acknowledge the variability in the results. Some studies did not observe significant differences between PDT in combination with conventional treatment and conventional treatment alone [10,13,15,16,28]. This variability could be attributed to several factors, including differences in study design, patient populations, PDT protocols, PSs used, and light parameters. These variations highlight the need for standardisation and consistency in future research to obtain more robust and generalisable results.
In the analysis of the 21 studies included in present review, it is noteworthy that one study was identified as having a high-risk of bias, as indicated by the risk of bias assessment tool utilised [13]. This finding emphasises the importance of critically evaluating the methodological quality of studies in research synthesis, as studies with a high-risk of bias may introduce substantial uncertainty and potential inaccuracies in the conclusions drawn. Additionally, the discovery that six studies demonstrated an overall unclear risk of bias further underscores the need for transparent reporting and robust methodological approaches in future research endeavours. Addressing and minimising bias in study design, conduct, and reporting are imperative to enhance the reliability and validity of research outcomes, ultimately contributing to evidence-based decision-making in healthcare and clinical practice.
Factors affecting PDT efficacy: The review identified various factors that can influence the efficacy of PDT in periodontal disease management. One crucial aspect is the selection of appropriate PSs and their concentrations. The PSs should possess the following properties: a high binding affinity for the target microorganism, a broad spectrum of action, a low binding affinity for mammalian cells to avoid the risk of photodestruction of host tissues, a low propensity for selecting resistant bacterial strains, a minimal risk of promoting mutagenic processes, and low chemical toxicity [32].
Different PSs exhibit varying levels of bacterial selectivity and activation wavelengths, which can impact the overall effectiveness of PDT. Generally, Gram-positive bacteria are susceptible to photo-inactivation, whereas Gram-negative bacteria are often resistant unless the permeability of their outer membrane is modified. This is connected to the difficulties encountered by PSs in penetrating gram negative bacterial cells. Antimicrobial PSs such as porphyrins, phthalocyanines, and phenothiasines (e.g., methylene blue and toluidine blue O) have been reported to penetrate both gram-positive and gram negative bacteria. The positive charge seems to promote the binding of the PSs to the gram negative bacterial membrane, leading to localised damage and resulting in increased permeability. Hence, toluidine blue O and methylene blue are commonly used in antimicrobial photodynamic therapy (aPDT). The hydrophilicity, low molecular weight, and positive charge of methylene blue facilitate its passage across the porin-protein channels in the gram negative outer bacterial membrane. Methylene blue’s interaction with the anionic lipopolysaccharide macromolecule of gram negative bacteria results in the generation of methylene blue dimers, which participate in the photosensitisation process [32,33].
Moreover, the choice of light sources and their parameters, including light intensity and exposure time, can also influence the photodynamic reaction [34]. Therefore, optimising these parameters is essential for achieving consistent and reproducible outcomes in PDT-based periodontal therapies.
Safety and adverse effects: The safety profile of PDT in periodontology was explored in the review. PDT is generally considered safe, with minimal adverse effects reported in the selected studies [35-38]. However, like any medical procedure, PDT is not entirely devoid of risks. Potential adverse effects may include mild discomfort, tissue sensitivity to light, and transient post-treatment inflammation [39]. Nonetheless, the incidence of serious complications is low, indicating that PDT can be considered a safe treatment modality when appropriately administered. Long-term studies with larger sample sizes would be beneficial to further assess the safety and potential long-term effects of PDT in periodontal patients.
Future perspectives and recommendations: The systematic review has highlighted the potential of PDT as a valuable addition to periodontal disease management. However, several avenues for future research and improvements in PDT’s clinical application have been identified. Further investigations are needed to establish the long-term efficacy of PDT and to identify optimal treatment protocols, including the most suitable PSs and light parameters [40]. Large-scale randomised controlled trials and comparative studies can provide stronger evidence for the effectiveness of PDT and enable the identification of specific patient populations that may benefit the most from this therapy. Additionally, exploring the use of PDT in combination with other emerging periodontal treatments or technologies may offer further benefits and enhance its efficacy.
Limitation(s)
Studies on PDT in periodontology may vary widely in terms of study designs, patient populations, intervention protocols, and outcome measures, making it challenging to draw direct comparisons or generalise findings and many studies may have short-term follow-up periods, which limits the assessment of the long-term efficacy and safety of PDT in periodontal treatment.
Conclusion(s)
The PDT presents a promising scope in periodontology, showcasing varied roles ranging from antimicrobial action to tissue healing and periodontal health development. Its efficacy as an adjunctive treatment, especially in challenging cases or against resistant microbes, is evident. This is further accentuated by its non invasive nature and minimal adverse effects, making it an appealing option in periodontal care. Despite these advantages, PDT’s full potential remains untapped due to challenges such as protocol standardisation, optimising light sources, and identifying ideal PSs, which necessitates further investigation. Moreover, addressing cost-effectiveness and accessibility concerns is pivotal for the widespread adoption of PDT. In essence, PDT offers a pathway for advancements in periodontal therapy. Ongoing research and trials are vital to unravel its mechanisms, improve protocols, and solidify its role in enhancing periodontal treatment outcomes.
SRP: Scaling and root planning; aPDT: Anti-microbial photodynamic therapy; PDT: Photodynamic therapy; CAL: Clinical attachment level; PPD: Probing pocket depth; PI: Plaque index; GI: Gingival index; MeSH: Medical subject headings
1BOP: Bleeding on probing; 2PPD: Pocket probing depth; 3CAL: Clinical attachment loss; 4FMPS: Full mouth plaque score; 5FMBS: Full mouth bleeding score; 6RAL: Relative attachment loss; 7PI: Plaque index; 8PD: Probing depth; 9GR: Gingival recession; 10GI: Gingival index; 11GBI: Gingival bleeding index; 12SBI: Sulcular bleeding index; 13FMPI- full mouth plaque index; 14FMBOP: Full mouth bleeding on probing; 15mSBI-modified sulcular bleeding index; 16GI: Gingival index simplified; 17PCR: Plaque control record