Case Report
A 30-year-old staff nurse presented to the Emergency Medical Service (EMS) with an alleged history of a snake bite on the anterior aspect of her neck two hours prior to arrival. She reported shooting pain associated with mild swelling. She also complained of a headache and giddiness. Pain at the bite site developed, for which she was given Tab. Paracetamol (500 mg). Based on her description, the snake was identified as a Russell viper. The bite site exhibited swelling and fang marks, but there was no active bleeding. Upon arrival, she showed no signs of envenomation. She had a past medical history of a vallecular cyst and underwent tracheostomy surgery 13 years ago. She had no history of any pre-existing comorbidities or significant family history. Therefore, she was kept under observation for further signs and symptoms.
During her observation period, the patient was haemodynamically stable, with a Blood Pressure (BP) of 100/60 mmHg and a Pulse Rate (PR) of 69 beats per minute. On examination, the patient’s pupils were dilated to about 4 mm, with normal extraocular movements. Within one hour of arrival, the patient suddenly developed breathlessness, chest tightness, and palpitations. Her BP rose to 160/100 mmHg, and her PR increased to 152 beats per minute. The monitor showed Supraventricular Tachycardia (SVT) waves, so carotid massage was administered, and the patient reverted back to normal. Later, the patient again experienced similar episodes within 30 minutes and became unresponsive. Although her eyes were open and she exhibited eye blinking, she was unable to respond to commands and showed generalised flaccidity, brisk reflexes, and a mute bilateral plantar response. Her vitals were monitored and remained similar to the earlier readings. Then, within five to 10 minutes, the patient recovered spontaneously.
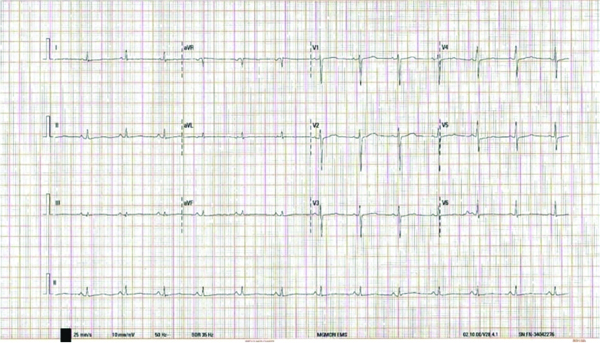
According to the standard treatment guidelines for the management of snake bites in India [1], a 20-minute Whole Blood Clotting Test (WBCT) was conducted on admission, which indicated that blood clotted in less than 20 minutes, ruling out an incoagulable state. Consequently, ASV was administered immediately, consisting of eight vials, each containing 10 mL. Routine blood and urine investigations were performed on admission [Table/Fig-1] and yielded normal results. Her renal and liver profiles were normal, except for elevated Aspartate Transaminase (AST) levels [Table/Fig-1]. Prothrombin Time (PT) was 15.4 seconds, and activated Partial Thromboplastin Time (aPTT) was found to be low at 24.5 seconds [Table/Fig-2]. Additionally, Electrocardiography (ECG) and Echocardiography (ECHO) showed no abnormalities [Table/Fig-3,4].
Initial laboratory investigations during admission.
| Investigations | Results | Reference range | Units |
|---|
| Total White Blood Cells (WBC)count | 6800 | 4000-11000 | cells/mm3 |
| Total Red Blood Cell (RBC) count | 3.99 | 4.5-5.5 | million/mm3 |
| Haemoglobin (Hb) | 11.3 | 13-17 | g/dL |
| Packed Cell Volume (PCV) | 34.1 | 40-50 | % |
| Mean Corpuscular Volume (MCV) | 86 | 83-101 | fL |
| Mean Corpuscular Haemoglobin (MCH) | 28.4 | 27-32 | pg |
| Mean Corpuscular Haemoglobin Concentration (MCHC) | 33.3 | 31.5-34.5 | % |
| Red Blood Cell Distribution Width (RDW-CV) | 13.5 | 11.5-14.5 | % |
| Platelet count | 253000 | 15000-450000 | lakhs/mm3 |
| Glucose | 97 | Up to 170 | mg/dL |
| Differential counts |
| Neutrophils | 38.5 | 40-75 | % |
| Lymphocytes | 52.8 | 20-45 | % |
| Eosinophils | 1.4 | Up to 6 | % |
| Basophils | 0 | Up to 2 | % |
| Monocytes | 7.3 | 2-10 | % |
| Microbiology |
| HbsAg rapid card test | Negative | | |
| HCV rapid card test | Non-reactive | | |
| Electrolytes |
| Sodium (Na+) | 139 | 135-145 | mEq/L |
| Potassium (K+) | 3.9 | 3.5-5.4 | mEq/L |
| Chloride (Cl-) | 110 | 98-107 | mEq/L |
| Live and renal function test |
| Blood urea | 19 | 15-45 | mg/dL |
| Serum creatinine | 0.80 | 0.6-1.2 | mg/dL |
| Total protein | 6.8 | 6-8.3 | g/dL |
| Albumin | 4.3 | 3.7-5.3 | g/dL |
| Globulin | 2.5 | 2.3-3.6 | g/dL |
| A/G ratio | 1.7:1 | | |
| Total bilirubin | 0.5 | 0.2-1 | mg/dL |
| Direct bilirubin | 0.2 | Up to 0.2 | mg/dL |
| Indirect bilirubin | 0.3 | 0.2-0.6 | mg/dL |
| SGOT (AST) | 44 | Up to 37 | U/L |
| SGPT (ALT) | 26 | Up to 40 | U/L |
| Alkaline phosphatase | 91 | 60-170 | U/L |
SGOT (AST): Serum glutamic-oxaloacetic transaminase (aspartate aminotransferase); SGPT (ALT): Serum glutamic pyruvic transaminase (Alanine aminotransferase); A/G: Albumin/globulin
Coagulation properties of the patient at the time of admission.
| Investigations | Results | Reference range | Units |
|---|
| Platelet count | 2.56 | 1.50-4.50 | lakhs/mm3 |
| Prothrombin Time (PT) | Test | 15.4 | | sec |
| Control | 13.5 | | sec |
| INR | 1.15 | 1-1.5 | |
| APTT | Test | 24.5 | 30-40 | sec |
| Control | 32.0 | 12-60 | sec |
APTT: Activated partial thromboplastin clotting time; INR: International normalised ratio
Electrocardiography (ECG) report of the patient- Heart rate 75/min, normal sinus rhythm, normal cardiac axis, and no dynamic ST-T changes.
Imaging findings of the patient.
| ECHO | Normal cardiac chamber dimensions, no RWMA, LVEF-60%, LVFP-11.5, PASP ~35 mmHg, trivial mitral and tricuspid regurgitation, normal pulmonary valve, no clot/pericardial effusion. |
| EEG | Scattered sharp waves and high voltage slow waves from both cerebral hemispheres suggestive of generalised epileptiform discharges. |
| MRI of brain | Normal Brain neuroparenchyma and no evidence SOL/Infarct/haemorrhage noted. |
ECHO: Echocardiography; EEG: Electroencephalography; RWMA: Regional wall motion abnormality; LVEF: Left ventricular ejection fraction; LVFP: Left ventricular filling pressure; PASP: Pulmonary artery systolic pressure; MRI: Magnetic resonance imaging; SOL: Space occupying lesions
The patient was then shifted to the Medical Intensive Care Unit (MICU) for further observation. After four hours, she reported experiencing nausea and anxiety and had a panic attack, during which she briefly lost consciousness and exhibited confusion, teeth clenching, and irregular movements that lasted for about 30 to 40 seconds. A psychiatric consultation was sought for a transient anxiety state, and the patient was counselled and reassured. Despite this, the patient continued to experience two to three similar episodes daily. Consequently, a Computed Tomography (CT) scan and MRI of the brain were performed to identify potential demyelination secondary to envenomation, but the results were normal [Table/Fig-4]. A neurologist’s opinion was obtained due to the recurrent episodes, and an EEG was advised.
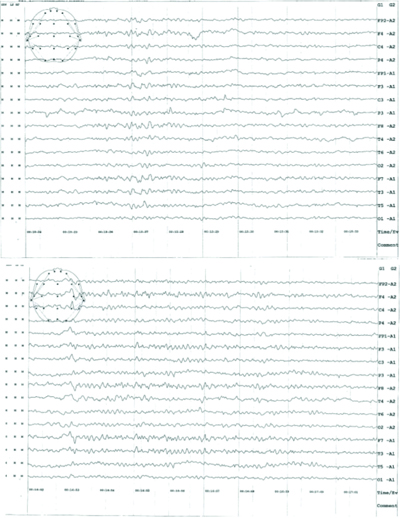
Within 24 hours, the patient underwent an EEG [Table/Fig-5], which revealed generalised epileptiform activity (bursts of sharp waves from both cerebral hemispheres). She was diagnosed with idiopathic epilepsy and was started on intravenous Levetiracetam 500 mg twice daily (an antiseizure medication). Levetiracetam was continued until her epileptic spells were under control. Although, she was advised to undergo an MRI, she was unable to do so due to financial constraints. Her prognosis improved, and she was discharged after three days with oral antiseizure medications and a follow-up plan.
Electroencephalography of the patient- Both the record shows burst of high voltage slow wave discharges from both cerebral hemispheres suggestive of epileptiform discharges.
After one month of being seizure-free, she was readmitted due to experiencing multiple episodes of seizures, each lasting about 40 to 50 seconds. Upon admission, a contrast MRI of the brain was repeated, which revealed normal neuroparenchyma. These findings led to a diagnosis of stress-induced late-onset adult idiopathic epilepsy. Following the neurologist’s opinion, she was administered inj. Lacosamide 50 mg BD (an antiseizure agent), but there was no improvement in the frequency or duration of the seizures. Subsequently, another antiseizure medication, tab. Lamotrigine (50 mg), was added, resulting in a significant response with no further epileptic spells. The patient had a good prognosis. After 10 days, she was discharged, and her injections were converted to oral medication with a titrated dosage of tab. Lacosamide 50 mg BD and tab. Lamotrigine 100 mg BD. She was advised to have regular follow-ups on an outpatient basis, and an EEG was performed to monitor the findings. The medication was tapered slowly after no changes were observed in the EEG.
Discussion
Snake bites are medical emergencies influenced by the bite site, snake type, and venom [1,2]. Russell’s viper, Daboia russelii, is a highly venomous terrestrial snake of the family Viperidae, commonly found in southern India [3,4]. Its venom contains various proteins and procoagulant enzymes that activate the coagulation cascade, leading to the consumption of clotting factors by activating factors V, IX, X, and XIII, and subsequently forming intravascular fibrin thrombi [2,4,5]. Due to its haemotoxic properties, it causes bleeding and coagulation disorders, which alter PT and aPTT [1,3]. The patient in this case also had a low aPTT. Snake envenomation is a serious issue that requires immediate treatment and can present with potentially fatal outcomes [1,6,7]. Unfortunately, it remains neglected in many tropical and subtropical countries [8]. Many published reports indicate that India has the highest mortality rate (81,000-138,000 people per year) due to snake bites [6,7]. However, many unreported cases occur due to non-medical treatment-seeking behaviour [7].
Common manifestations include local bleeding, swelling, necrosis, pain at the bite site, and signs of systemic bleeding, such as gingival bleeding, epistaxis, ecchymosis, haematemesis, haemoptysis, subconjunctival haemorrhage, haematochezia, and bleeding from pre-existing conditions [1]. In this patient, symptoms of envenomation began at the affected extremity with pain, followed by swelling and mild bleeding; however, the characteristic features of haemotoxicity were not presented.
Epilepsy in Russell’s viper is considerably rare. A study conducted by Huang YK et al., showed that D. russelii, which belongs to the Viperidae family, presents with both haemotoxic and neurotoxic venoms that can cause neural disorders [9]. These disorders include ptosis, seizures, hypotonia, and other neurological issues. However, in the present case, generalised epileptiform activity occurred four hours after the snakebite, despite a normal brain MRI with no evidence of Intracranial Haemorrhage (ICH) and a normal EEG. This led to the assumption of idiopathic epilepsy, particularly as the patient had no prior history of seizures.
Abnormal electrical activity in the brain can be caused by various factors, including electrical shock, head injury, tumours, infections, strokes, and venomous bites with neurotoxic potential, as well as withdrawal from alcohol and other causes [10]. Individuals experiencing seizures often have warning symptoms before an attack, such as flashing lights, anxiety, fear, vertigo, and nausea, which can lead to intractable epilepsy [11,12]. In this case, the presence of haemotoxic venom, without ICH, resulted in a rare presentation. However, there are no case reports supporting the idea that viper venom can cause idiopathic epilepsy, making this case particularly unusual. In [Table/Fig-6], a few cases that exhibited neurological findings after snakebite are summarised [2,4,5,8,13].
Review of the neurological presentation by Viper venom [2,8,4,5,13].
| Authors name and year [Reference] | Place | Age/Sex of patient | Unusual presentation seen after snake bite | Management and prognosis |
|---|
| Seo YH et al., 2014 [13] | Korea | 44/F | Complex regional pain syndrome | Gabapentin 300 mg and discharged. Recovered. |
| Puri S et al., 2014 [4] | India | 36/M | Generalised seizures | ASV, tetanus toxoid, antibiotics, anti-oedema measures and anti-platelets. Recovered and discharged. |
| 40/M | Broca’s aphasia | ASV with supportive treatment. Residual dysfunctions were present. Recovered. |
| Lahiri D et al., 2019 [5] | India | 50/M | Status epilepticus and bilateral middle cerebral artery infarction with global aphasia | Aphasia was improved yet, motor deficit on right were persistent at the time of follow-up. |
| Banerjee S et al., 2019 [2] | India | 40/F | Cerebellar ataxia | ASV and symptomatic treatment. Not recovered and lost to follow-up. |
| Yousaf M et al., 2023 [8] | Pakistan | 25/M | Cerebral venous sinus thrombosis | ASV and with rivaroxaban and levetiracetam. Recovered. |
Conclusion(s)
The case report highlights the importance of early detection and treatment of stress-induced late-onset adult epilepsy following a snakebite. It also emphasises the need for a high index of suspicion and early detection to reduce morbidity and mortality, especially in individuals with no prior history of epileptic episodes.
SGOT (AST): Serum glutamic-oxaloacetic transaminase (aspartate aminotransferase); SGPT (ALT): Serum glutamic pyruvic transaminase (Alanine aminotransferase); A/G: Albumin/globulin
APTT: Activated partial thromboplastin clotting time; INR: International normalised ratio
ECHO: Echocardiography; EEG: Electroencephalography; RWMA: Regional wall motion abnormality; LVEF: Left ventricular ejection fraction; LVFP: Left ventricular filling pressure; PASP: Pulmonary artery systolic pressure; MRI: Magnetic resonance imaging; SOL: Space occupying lesions
[1]. Management of Snake Bite [Internet][cited 2024 Jan 31]. Available from: https://qps.nhsrcindia.org/sites/default/files/2021-05/Management%20of%20Snake%20Bite.pdf [Google Scholar]
[2]. Banerjee S, Pipliwal PS, Joshi S, Goyal P, Sharma M, Sharma A, Cerebellar ataxia: A rare manifestation of snake envenomationJ Indian Med Assoc 2019 117(6):28-29. [Google Scholar]
[3]. Laxme RRS, Khochare S, Attarde S, Suranse V, Iyer A, Casewell NR, Biogeographic venom variation in Russell’s viper (Daboia russelii) and the preclinical inefficacy of antivenom therapy in snakebite hotspotsPLoS Negl Trop Dis 2021 15(3):e000924710.1371/journal.pntd.000924733764996 [Google Scholar] [CrossRef] [PubMed]
[4]. Puri S, Paul G, Paul BS, Snake bite and stroke: Our experience of two casesIndian J Crit Care Med 2014 18(4):257-58.10.4103/0972-5229.13058524872661 [Google Scholar] [CrossRef] [PubMed]
[5]. Lahiri D, Sawale VM, Dubey S, Roy BK, Das SK, Status epilepticus and bilateral middle cerebral artery infarction: A rare presentation after viper biteAnn Afr Med 2019 18(2):111-14.10.4103/aam.aam_21_1831070155 [Google Scholar] [CrossRef] [PubMed]
[6]. WHO/SEARO Guidelines for the clinical management of snake bites in the Southeast Asian regionSoutheast Asian J Trop Med Public Health 1999 30(Suppl 1):01-85. [Google Scholar]
[7]. Snakebite envenoming India [Internet][cited 2023 Sep 24]. Available from: https://www.who.int/india/health-topics/snakebite [Google Scholar]
[8]. Yousaf M, Khan QA, Anthony MR, Badshah A, Abdi P, Farkouh C, Snakebite induced cerebral venous sinus thrombosis: A case reportClin Med Insights Case Rep 2023 16:1179547623116575010.1177/1179547623116575037033678 [Google Scholar] [CrossRef] [PubMed]
[9]. Huang YK, Chen YC, Liu CC, Cheng HC, Tu AT, Chang KC, Cerebral complications of snakebite envenoming: Case studiesToxins (Basel) 2022 14(7):43610.3390/toxins1407043635878174 [Google Scholar] [CrossRef] [PubMed]
[10]. Chauhan V, Thakur S, The North–South divide in snake bite envenomation in IndiaJ Emerg Trauma Shock 2016 9(4):151-54.10.4103/0974-2700.19335027904261 [Google Scholar] [CrossRef] [PubMed]
[11]. Youmans and Winn Neurological Surgery: 8th edition. H. Richard Winn. [Internet]Elsevier Asia Bookstore[cited 2024 Jan 31]. Available from: https://www.asia.elsevierhealth.com/youmans-and-winn-neurological-surgery-9780323661928.html [Google Scholar]
[12]. Cutting S, Lauchheimer A, Barr W, Devinsky O, Adult-onset idiopathic generalized epilepsy: Clinical and behavioural featuresEpilepsia 2001 42(11):1395-98.10.1046/j.1528-1157.2001.14901.x11879340 [Google Scholar] [CrossRef] [PubMed]
[13]. Seo YH, Park MR, Yoo SH, Development of complex regional pain syndrome after a snake bite: A case reportKorean J Pain 2014 27:68-71.10.3344/kjp.2014.27.1.6824478904 [Google Scholar] [CrossRef] [PubMed]