Introduction
Microscopes are instruments that magnify the images of very small objects, such as cells and microorganisms and are primarily used in science laboratories. A light microscope is one of the important tool for research conducted by cell biologists. Until recently, ultra-structural details could not be observed using light microscopes due to their low resolution. However, advancements in optical designs have opened new opportunities for microscopists to better understand and study cellular structures with greater visibility.
There are various types of microscopes, depending on the source of light used and the types of lenses involved. The confocal microscope is one such type, which uses lasers as a light source. In recent times, confocal microscopes have become available to researchers with advanced optical designs. Modern confocal microscopes offer increased optimised resolution, better differentiation and greater depth of field. Confocal microscopy allows for rapid, non invasive imaging of thick samples, producing high-resolution images and three-dimensional structures, especially in comparison to electron microscopy [1].
The present article provides a detailed overview of the principles of confocal microscopy, including its various applications, advantages and limitations associated with the laser scanning confocal microscope.
The CLSM is a type of fluorescence microscopy that scans a focused point of laser light in a diffraction manner around a sample to produce high-resolution images of objects stained with fluorescent probes [1]. The term “confocal” refers to having the same focus, meaning that the image plane and the confocal pinhole are conjugate. This configuration eliminates light from above and below the plane of focus, resulting in a crisp image with high resolution.
The original concept of confocal microscopy was developed by Marvin Minsky, who experimented with neural networks to view unstained preparations of brain tissue. However, the invention went unnoticed due to poor light sources [2]. Later, in the late 1970s and 1980s, significant improvements in computer and laser technology, along with the availability of digital image manipulation, led to a marked increase in interest in confocal microscopy [3,4].
Modern confocal microscopes are equipped with powerful lasers, wavelength selection options, one or more electronic detectors and a beam scanning assembly. The confocal microscope works by integrating all these components to produce a digital image. This advanced microscopy allows for the imaging and investigation of living tissues, cells and molecules, which were previously impossible [5].
Reflectance confocal microscopy is another type of optical imaging technique that provides a horizontal view of the specimen. It uses laser light at near-infrared wavelengths, which penetrates the tissue to illuminate a single spot [6-8].
Though there are many types of confocal microscopes, including laser scanning microscopy, spinning disk microscopes, programmable array microscopes and swept field microscopes, the present article mainly focuses on the laser scanning confocal microscope.
Search Methods
A search was conducted in electronic databases, including PubMed, Scopus, Web of Science and Google Scholar. The search terms included “confocal microscopy”, “CLSM,” and related variations. The inclusion criteria encompassed studies using CLSM that were published in peer-reviewed journals and available in English.
Study selection was performed after removing duplicates and assessing the eligibility of articles based on their titles, abstracts and full texts. Data extraction included study characteristics, CLSM applications, technical advancements and limitations. The extracted data were synthesised and analysed to provide a comprehensive overview of CLSM.
Principle of Confocal Microscopy
The basic principle of confocal microscopy is the generation of a high-resolution image using a focused spot of laser light directed through an objective lens onto a specific object plane of interest. Confocal microscopy also employs fluorescence optics, like wide-field fluorescence microscopy. In wide-field microscopy, the entire sample is illuminated at once, while in confocal microscopy, laser light is focused on a diffraction-limited spot at a specific depth within the sample [2,4].
Point illumination directs light precisely to a specific point, while out-of-focus light is eliminated by a pinhole located within the optical pathway. As a result, only fluorescence signals from the focused spot reach the light detector, forming a sharp image [Table/Fig-1].
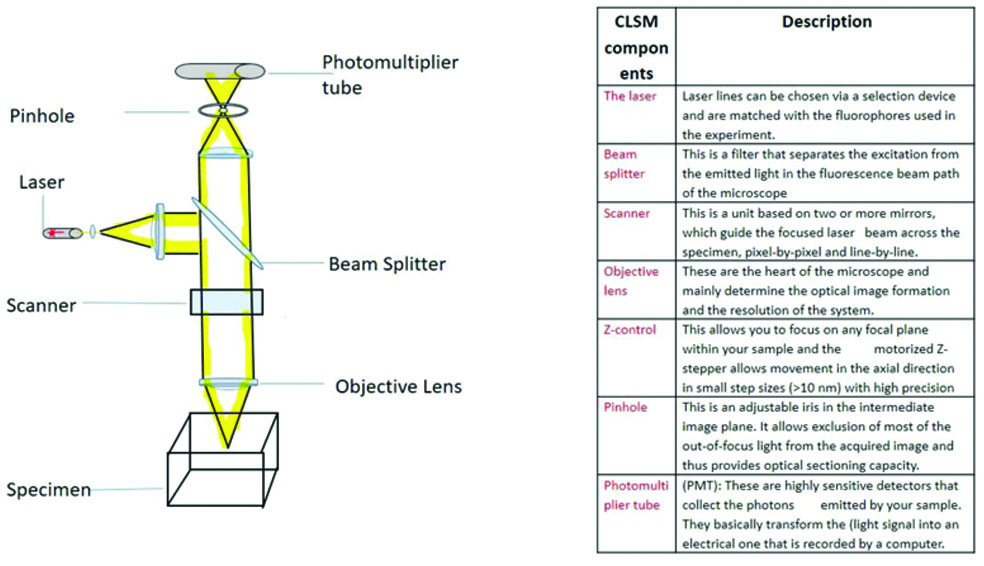
The generation of CLSM images involves three process steps. Fluorescent molecules in the specimen are excited by laser light, resulting in fluorescence. The emitted fluorescent light is refocused in the objective image plane and out-of-focus light is removed from the image by passing the light through a pinhole. The fluorescence emitted by the scanned specimen is detected pixel by pixel with a Photomultiplier Tube (PMT), where a pixel represents a picture element. To capture a sharp image, the size of the confocal pinhole should be matched with the size of the Airy disk. The Airy disk is the central diffraction spot of light, having a finite diameter enclosed by the first minimum of the airy pattern, named after British astronomer GB airy [4]. At one airy unit, the system is diffraction-limited [4,5]. The pinhole may be opened for dim samples, but this comes at the cost of resolution. The brightness of the image depends on the number of photons acquired. Image resolution is improved with a higher number of pixels and a larger scanning area can be covered at a given pixel size [9,10]. The resolution is defined as the smallest distance between two points on a specimen that can be distinguished as two separate entities by an objective, while the numerical aperture refers to the light-gathering capacity of the objective.
Scanning speed is equally important in confocal imaging. A slower scanning speed can lead to photobleaching of the specimen, while increased speed decreases the pixel dwell time- the amount of time the laser spends on each pixel- thereby reducing the signal-to-noise ratio [5,11].
In a confocal microscope, different modes of light microscopy imaging can be collected using the transmitted light detector, which includes bright field, differential interference contrast and others. Images captured can be further deconvolved using software such as “Image J”, where deconvolution is an image processing method for reducing blurring in fluorescent imaging. This technique improves the contrast and resolution of the image through mathematical operations. However, deconvolution should be applied carefully and accurately [12].
Components of Confocal Laser Scanning Microscope
The components of a CLSM are depicted in [Table/Fig-1] and are expanded upon here. The laser serves as the source of light for the confocal microscope. The available laser lines depend on the model of the confocal microscope. The fluorophores used in the experiment are chosen according to the available laser lines. An ultraviolet laser range can be installed if it is not already provided with the confocal microscope. A beam splitter or dichroic mirror separates the excitation light from the emitted light in the fluorescence beam path of the microscope.
The scanning of the laser beam is guided by two or more scanning mirrors. The confocal microscope scans the sample point by point in a raster pattern. The objective lens of the microscope determines the optical image formation and resolution of the image. Among the various types of objectives, plan apochromatic objective lenses are considered the best type, as they correct for both spherical and chromatic aberrations. The pinhole present in the system excludes out-of-focus light from the focal plane, resulting in a sharper image. It is measured in airy units, with one airy unit being considered optimal for imaging. This measurement is calculated as the resolution of the lens multiplied by the magnification of the lens, multiplied by two.
The PMTs are the detectors that convert photons emitted from the sample into electrical signals. These detectors are very sensitive, fast and possess a large dynamic range. However, the quantum efficiency of PMTs is not as high when compared to Charge-Coupled Devices (CCDs), which can respond to incoming photon signals and convert them to measurable electron signals [4,5]. Hybrid detectors are also used as an alternative means to overcome the photobleaching of the fluorescent probe [13,14].
Sample Preparation
Sample preparation is a crucial step in confocal imaging. It includes the proper fixation of the sample, permeabilisation of tissue or cells for targeted intracellular antigen detection and labelling methods that preserve cellular morphology. Fixatives and permeabilising agents should be chosen according to the type of cells to be visualised, as each fixative or permeabilising agent has different advantages and drawbacks. When selecting a fluorophore, factors such as the quantum efficiency of the detector and the excitation and emission spectra of the probe should be considered [11].
The mounting medium should be chosen to maintain the desired information while providing significant signal strength, including antifading properties. Care should be taken to prevent damage to the sample. Coverslips are then sealed using colourless nail polish to avoid drying out the specimen. The specimen may be preserved in the dark in the refrigerator until acquisition [11,12].
Advantages and disadvantages of confocal microscopy [Table/Fig-2].
Advantages and limitations of confocal microscope.
| S. No. | Advantages of confocal microscopy | Limitations of confocal microscopy |
|---|
| 1 | High-resolution image, 3D images of the specimen by collecting serial optical sections. | Limited number of excitation wavelengths |
| 2 | Living and fixed cells can be used.Magnification is adjusted electronically by zoom factor. | Expensive to produce the ultraviolet rays used by the Confocal Microscopes and expensive to purchase |
| 3 | Very high sensitivity and better resolution with uniform scanning. | Phototoxicity of used probe |
In confocal microscopy, out-of-focus light is suppressed during image formation, increasing axial resolution. The signal-to-noise ratio is improved in confocal imaging, making it suitable for both living and fixed cell imaging. Optical sections of thick specimens are collected in a confocal microscope using motorised Z-axis movement. The confocal laser scanning microscope generates various images of the Z focal planes through optical sectioning, which are referred to as a z-series. These images are collected by moving the specimen only along the Z-axis while keeping the position fixed (with no movement in the X-Y axis). The resulting data can be viewed as three-dimensional multicolour video sequences in real-time.
Time-lapse and live cell imaging are additional advantages of confocal microscopy. The single optical sections captured at preset points in time constitute time-lapse images. Time-lapse images are used to study the dynamics of living cells with increased resolution. The magnification of the confocal microscope can be adjusted electronically without changing the objectives by varying the scanned area. This feature is called the zoom factor and is used to adjust image resolution [12].
The disadvantages of confocal microscopy include the limited number of available excitation wavelengths, which do not encompass the ultraviolet range. However, this limitation can be addressed by installing a white light supercontinuum laser as a versatile substitute for the multiple monochromatic lasers typically used for fluorescence excitation, particularly in live cell imaging [10]. Confocal microscopes are comparatively expensive to purchase but can serve as centralised instruments for various departments. Phototoxicity of the probe is another disadvantage of CLSM, but hybrid detectors are available to mitigate photobleaching and monitor protein interactions in living cells [13-16].
Confocal microscopes have various applications in cell biology as well as in the food industry. These applications include techniques such as Fluorescence Recovery After Photobleaching (FRAP), Fluorescence Resonance Energy Transfer (FRET) and Fluorescence In-situ Hybridisation (FISH). FRAP is a technique used to study protein mobility in living cells; a specific area of a cell or tissue is photobleached using intense laser light and the recovery of fluorescence in that area is monitored.
The FRET allows for the determination of the proximity between two molecules within several nanometers, a distance sufficiently close for molecular interactions to occur. FRET can be employed to map protein-protein interactions. LysoTracker Red is a probe used to study apoptosis, which can be visualised using confocal microscopy [17,18]. In-vivo corneal confocal microscopy is gaining importance in the diagnosis of ocular diseases as it describes changes in corneal nerves and sublayers because of z-sectioning ability [19,20]. FISH detects specific Deoxyribonucleic Acid (DNA) sequences on chromosomes. FISH, when combined with CLSM, is also used for analysing biofilm systems in wastewater treatment research [21]. Green Fluorescent Protein (GFP) is utilised to study gene expression and intracellular signalling.
In food technology, CLSM is employed to monitor dynamic processes as they relate to various function of variables in the food industry. Changes in temperature, pH, concentration and pressure are monitored using fluorophores, which are crucial factors in the transportation of food products [22]. Commonly consumed wheat products, such as bread, wheat dough and pasta, are imaged using CLSM with fluorescent dyes, allowing for the acquisition of three-dimensional information on the cellular structure of yam parenchyma and on the properties of protein and starch networks in wheat products [23]. Reflection mode analysis enables the examination of surface properties of pasta.
Live cell imaging using CLSM is an efficient method for studying the physiology and pathophysiology of cells, including calcium dynamics. Calcium signals observed using CLSM can be detected in a large number of endocrine and exocrine cells with single-cell or even subcellular resolution [24-26].
The applications of CLSM have a wide range, including but not limited to:
Cell Biology: Visualisation of cellular structures, organelles and dynamic processes in living cells [23].
Neuroscience: Imaging of neuronal morphology and neural circuitry [24].
Materials science: Analysis of surface topography, microstructure and defects in materials [25].
Medicine: Diagnosis and monitoring of diseases, including cancer and ophthalmic disorders [27].
Environmental Science: Study of microorganisms and particulate matter in environmental samples [28].
Key limitations of CLSM include:
Photobleaching and phototoxicity
Limited penetration depth in thick specimens
Equipment cost and complexity
Data processing and storage requirements
Potential Uses of Confocal Microscopy
The confocal microscope is beneficial in a wide range of fields, including fundamental and medical research, nanoparticle imaging and quantum optics.
In the medical field, corneal confocal microscopy is widely used to diagnose ocular diseases. This modern, non invasive In-vivo ophthalmic imaging technique can accurately and rapidly measure the damage and repair of small fibers in Parkinson’s disease [27]. Confocal microscopy is also utilised to develop and explore automated cell identification and segmentation methods for the morphometry of corneal epithelial cells [28,29]. Another common application of confocal microscopy is the examination of surface and near-surface features in biomaterials such as bone, dentin and enamel.
Microbial resistance, particularly in hospitalised patients, is a growing concern. CLSM plays a vital role in detecting biofilms produced by microbes, aiding in the identification of antibiotic-resistant strains and redirecting treatment. In recurrent urinary tract infections, biofilms formed by uropathogenic gram-negative bacteria in urothelial cells are significant [30,31]. Microarray techniques combined with CLSM facilitate biofilm characterisation [32,33].
In tuberculosis, treatment challenges arise due to dormant mycobacteria. CLSM, paired with immunofluorescence, enhances the detection of M. tuberculosis in lung histological samples, especially when Ziehl-Neelsen staining is inadequate [34]. Dormant bacilli, often undetectable by routine methods, are crucial to study as they contribute to latent infections and prolonged therapy. CLSM improves TB diagnosis by visualising dormant bacilli and studying their metabolism, offering insights into drug and vaccine efficacy [34-36]. The optical sectioning feature of CLSM allows for precise identification of infected host cells and the location of bacilli, making it a valuable tool in medical research and clinical diagnostics [37].
Recently, due to the Coronavirus 2019 (COVID-19) pandemic, the study of coronaviruses has become an important aspect of treatment and vaccine development. Studies on the SARS-CoV-2 virus can be performed by fluorescently tagging or immunolabelling viral proteins or Ribooxynucleic Acid (RNA) strands. Fluorescence microscopy has been used in studies of different viruses, so it can also be applied to identify coronaviral infections [38]. To investigate the endocytic pathway used by the virus, confocal microscopy is employed in some studies. Recent research has revealed the role of a secretory form of the Angiotensin-converting Enzyme 2 (ACE2) receptor (sACE2) in SARS-CoV-2 infection [38,39]. The replicative cycle and immunopathogenic mechanisms of this novel coronavirus are being studied using several high-resolution microscopy techniques to develop new treatments against this disease [11]. In experimental studies, immunofluorescence staining reveals abundant ACE2, which is the SARS-CoV-2 receptor and Transmembrane Protease Serine 2 (TMPRSS2) protein in the cells of differentiated human enteroids [39].
In the case of cancer, therapy depends on the stages of the disease and can become difficult and painful in advanced stages. Early diagnosis is crucial for all types of cancer. Confocal microscopy is a useful technique that can be utilised for diagnosing various types of cancers. Many types of skin cancers, from melanoma to basal and squamous cell carcinomas, can be detected using confocal microscopy. This technique visualises epidermal cells and superficial dermal cells in real time manner [40,41]. It minimises the number of skin biopsies and can be applied both in-vivo and in-vitro. In basic dermatology, this microscopy is beneficial for clinical practice as well as basic research [42,43]. Confocal microscopy can monitor post-skin cancer lesions without the need for further biopsies, which provides patients with comfort and a more positive outlook about their care [44].
Cervical cancer is a preventable disease if diagnosed early. The diagnosis of cervical precancer can be improved using confocal microscopy, according to some studies. It has been observed that in the precancer state, epithelial fluorescence increases while stromal fluorescence decreases. By using the vital dye mitotracker orange, these changes can be observed. Confocal microscopy can provide information regarding epithelial changes through multiple images at various focal planes [45].
Nowadays, portable, automated models are available for in-vivo CLSM. In dermatology, this technology provides morphological characterisation of skin structures in the same area at different time points. It offers high-resolution images with a non invasive approach for real-time monitoring of diseases. Reflectance confocal microscopy is predominantly used for clinical diagnosis and research purposes in dermatology, while fluorescence confocal microscopy is also employed [44]. Portable models of confocal endomicroscopes are available for diagnosis as well. Cost-effective, convenient and robust models have been developed with automated alignment features. The clinical data obtained from such tools can be further evaluated. Nuclear morphology alterations in cervical lesions can be studied in real time, which aids in treatment decisions. Large-scale studies are necessary for further evaluation [44].
Currently, corneal confocal microscopy is gaining importance in the diagnosis of ocular diseases. Microbial keratitis is a common and blinding eye disease among contact lens wearers worldwide. In-vivo Confocal Microscopy (IVCM) has proven advantageous in the early diagnosis of fungal and Acanthamoeba keratitis. Disease monitoring is conducted according to the depth of Acanthamoeba cyst invasion, which informs antimicrobial therapy decisions. IVCM also assists clinicians in examining flap-related complications after refractive surgery and it describes changes in corneal nerves and sublayers [37]. Keratoconus, a corneal disease characterised by the development of a conical shape of the cornea, is diagnosed using confocal microscopy, where the loss of keratocytes is assessed by counting them with this technique.
Technological Advancements
Recent advancements in CLSM include:
Multi-photon microscopy: This technique offers enhanced penetration depth and reduced phototoxicity [45].
Super-resolution techniques: These methods improve spatial resolution beyond the diffraction limit [46,47].
Spectral imaging: This allows for the simultaneous acquisition of multiple fluorescent markers [48,49].
Light-sheet microscopy: This technique enables rapid imaging of large specimens with minimal photobleaching [49].
When combined with electron microscopy, confocal microscopy provides significant advancements in optical microscopy. Today, it is possible to achieve 4D imaging with new designs of confocal microscopy, capturing short-timescale dynamics. Such models include the LSM 980 with Airyscan, which allows for fast and gentle multiplex imaging.
Conclusion(s)
The CLSM is a very useful technique for imaging thick tissue samples. Considering some limitations of laser scanning microscopes, a technique that combines reflectance and fluorescence modes could be beneficial for obtaining additional information in various situations, such as the diagnosis of human skin morphology. More recently, live-cell confocal imaging has gained importance and portable models of confocal microscopes are now available for disease diagnosis. This technique is particularly useful for the early diagnosis of precancer stages in various types of cancers, helping to avoid repeated biopsies for the convenience of patients. The physiology and pathophysiology of various cell types can be observed using live-cell imaging in confocal microscopy. The CLSM remains a vital imaging technique with diverse applications across multiple scientific fields. Advancements in technology have expanded its capabilities, while ongoing research aims to overcome its limitations. As CLSM continues to evolve, it is likely to play an even more significant role in advancing our understanding of the micro and nano-scale world.
[1]. Elliott AD, Confocal microscopy: Principles and modern practicesCurr Protoc Cytom [Internet] 2020 92(1):e68Available from: https://dx.doi.org/10.1002/cpcy.6810.1002/cpcy.6831876974 [Google Scholar] [CrossRef] [PubMed]
[2]. Minsky M, Memoir on inventing the confocal scanning microscopyThe Journal of Scanning Microscopies 1988 10:128-38.10.1002/sca.4950100403 [Google Scholar] [CrossRef]
[3]. Song L, Varma CA, Verhoeven JW, Tanke HJ, Influence of the triplet excited state on the photobleaching kinetics of fluorescein in microscopyBiophys J [Internet] 1996 70(6):2959-68.Available from: https://dx.doi.org/10.1016/S0006-3495(96)79866-110.1016/S0006-3495(96)79866-18744334 [Google Scholar] [CrossRef] [PubMed]
[4]. Brian Matsumoto, Cell biological applications of confocal microscopyMethods in Cell Biology 2002 Vol. 702nd Edition [Google Scholar]
[5]. Pawley J, Handbook of Biological Confocal Microscopy 1995 New YorkPlenum Press10.1007/978-1-4757-5348-67485706 [Google Scholar] [CrossRef] [PubMed]
[6]. Bagc C, Sever M, Belder N, Bennett AP, Erdener S, Dalkaraa Overview of extracellular vesicle characterization techniques and introduction to combined reflectance and fluorescence confocal microscopy to distinguish extracellular vesicle subpopulationsNeurophotonics 2022 9:02190310.1117/1.NPh.9.2.02190335386596 [Google Scholar] [CrossRef] [PubMed]
[7]. Zeidan A, Yelin D, Reflectance confocal microscopy of red blood cells: Simulation and experimentBiomed Opt Express [Internet] 2015 6(11):4335-43.Available from: https://dx.doi.org/10.1364/BOE.6.00433510.1364/BOE.6.00433526600999 [Google Scholar] [CrossRef] [PubMed]
[8]. Skvara H, Plut U, Schmid JA, Jonak C, Combining invivo reflectance with fluorescence confocal microscopy provides additive information on skin morphologyDermatol Pract Concept [Internet] 2012 2(1):03-12.Available from: https://dx.doi.org/10.5826/dpc.0201a0210.5826/dpc.0201a02 [Google Scholar] [CrossRef]
[9]. Paddock S, Confocal Microscopy: Methods and Protocols 1999 Totowa, New JerseyHumana Press [Google Scholar]
[10]. Chiu LD, Su L, Reichelt S, Amos WB, Use of a white light supercontinuum laser for confocal interference-reflection microscopy: Supercontinuum for confocal interference reflectanceJ Microsc [Internet] 2012 246(2):153-59.Available from: https://dx.doi.org/10.1111/j.1365-2818.2012.03603.x10.1111/j.1365-2818.2012.03603.x22432542 [Google Scholar] [CrossRef] [PubMed]
[11]. Pawley CV, Tutorial on practical confocal microscopy and use of the confocal test specimenHandbook of Biological Confocal Microscopy 1995 New YorkPlenum Press10.1007/978-1-4757-5348-67485706 [Google Scholar] [CrossRef] [PubMed]
[12]. Lopez J, Fujii S, Doi A, Utsunomiya H, A deconvolution revolution for confocal image enhancementLaser Focus World 2019 55(1):85-88. [Google Scholar]
[13]. Foltánková V, Matula P, Sorokin D, Kozubek S, Bártová E, Hybrid detectors improved time-lapse confocal microscopy of PML and 53BP1 nuclear body colocalization in DNA lesionsMicrosc Microanal [Internet] 2013 19(2):360-69.Available from: https://dx.doi.org/10.1017/S143192761201435310.1017/S143192761201435323410959 [Google Scholar] [CrossRef] [PubMed]
[14]. Antolovic IM, Bruschini C, Charbon E, Dynamic range extension for photon counting arraysOpt Express [Internet] 2018 26(17):22234-48.Available from: https://dx.doi.org/10.1364/OE.26.02223410.1364/OE.26.02223430130919 [Google Scholar] [CrossRef] [PubMed]
[15]. Becker W, Su B, Holub O, Weisshart K, FLIM and FCS detection in laser-scanning microscopes: Increased efficiency by GaAsP hybrid detectorsMicrosc Res Tech 2011 74:804-11.10.1002/jemt.2095923939667 [Google Scholar] [CrossRef] [PubMed]
[16]. Mivelle M, Van Zanten TS, Garcia-Parajo MF, Hybrid photonic antennas for subnanometer multicolour localization and nanoimaging of single moleculesNano Letters 2014 14(8):4895-900.10.1021/nl502393b25050445 [Google Scholar] [CrossRef] [PubMed]
[17]. Zucker RM, Rogers JM, Confocal laser scanning microscopy of morphology and apoptosis in organogenesis-stage mouse embryosMethods Mol Biol [Internet] 2019 1965:297-311.Available from: https://dx.doi.org/10.1007/978-1-4939-9182-2_2010.1007/978-1-4939-9182-2_2031069683 [Google Scholar] [CrossRef]
[18]. Mukhopadhyay P, Rajesh M, Haskó G, Hawkins BJ, Madesh M, Pacher P, Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopyNat Protoc [Internet] 2007 2(9):2295-301.Available from: https://dx.doi.org/10.1038/nprot.2007.32710.1038/nprot.2007.32717853886 [Google Scholar] [CrossRef] [PubMed]
[19]. Zeiss 710 Inverted Confocal Manual. Light Microscopy Core Facility. Duke Trinity College of Arts and SciencesAvailable from: https://microscopy.duke.edu/manuals/zeiss-710-inverted-confocal-manual [Google Scholar]
[20]. Banayan N, Georgeon C, Grieve K, Ghoubay D, Baudouin F, Borderie V, Invivo confocal microscopy and optical coherence tomography as innovative tools for the diagnosis of limbal stem cell deficiencyJournal Français d’Ophtalmologie 2018 41(9):e395-406.10.1016/j.jfo.2018.09.00330458924 [Google Scholar] [CrossRef] [PubMed]
[21]. Jubany I, Lafuente J, Carrera J, Baeza J, Automated thresholding method (ATM) for biomass fraction determination using FISH and confocal microscopyJ Chem Technol Biotechnol 2009 84:1140-45.10.1002/jctb.2146 [Google Scholar] [CrossRef]
[22]. Dürrenberger MB, Handschin S, Conde-Petit B, Escher F, LWT-Food Science and TechnologyVisualization of Food Structure by Confocal Laser Scanning Microscopy (CLSM) 2001 34:11-17.10.1006/fstl.2000.0739 [Google Scholar] [CrossRef]
[23]. Cardona JAR, Iriart CH, Herrera ML, Applications of Confocal Laser Scanning Microscopy (CLSM) in FoodsIn (Ed.), Confocal Laser Microscopy-Principles and Applications in Medicine, Biology, and the Food Sciences 2013 IntechOpen [Google Scholar]
[24]. Stožer A, Dolenšek J, Križančić Bombek L, Pohorec V, Slak Rupnik M, Klemen MS, Confocal laser scanning microscopy of calcium dynamics in acute mouse pancreatic tissue slicesJ Vis Exp [Internet] 2021 (170)Available from: https://dx.doi.org/10.3791/6229310.3791/6229333938876 [Google Scholar] [CrossRef] [PubMed]
[25]. Wang JK, Zhao MQ, Sun NN, Sun FF, Wu J, Shen JX, Laser scanning confocal microscopic imaging for Ca2 + oscillations of pancreatic acinar cells in miceZhongguo Ying Yong Sheng Li Xue Za Zhi 2014 30(4):373-77. [Google Scholar]
[26]. Marolt U, Paradiž Leitgeb E, Pohorec V, Lipovšek S, Venglovecz V, Gál E, Calcium imaging in intact mouse acinar cells in acute pancreas tissue slicesPLoS One [Internet] 2022 17(6):e0268644Available from: https://dx.doi.org/10.1371/journal.pone.026864410.1371/journal.pone.026864435657915 [Google Scholar] [CrossRef] [PubMed]
[27]. Stachs O, Guthoff RF, Aumann S, High resolution imaging in microscopy and ophthalmology: New frontiers in biomedical optics. Bille JF, editor 2019 Cham (CH [Google Scholar]
[28]. Bhattacharya P, Edwards K, Schmid KL, Segmentation methods and morphometry of confocal microscopy imaged corneal epithelial cellsCont Lens Anterior Eye [Internet] 2022 45(6):101720Available from: https://dx.doi.org/10.1016/j.clae.2022.10172010.1016/j.clae.2022.10172035624027 [Google Scholar] [CrossRef] [PubMed]
[29]. Allgeier S, Bartschat A, Bohn S, Peschel S, Reichert KM, Sperlich K, 3D confocal laser-scanning microscopy for large-area imaging of the corneal subbasal nerve plexusSci Rep [Internet] 2018 8(1):7468Available from: https://dx.doi.org/10.1038/s41598-018-25915-610.1038/s41598-018-25915-629749384 [Google Scholar] [CrossRef] [PubMed]
[30]. Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ, Detection of intracellular bacterial communities in human urinary tract infectionPLoS Med [Internet] 2007 4(12):e329Available from: https://dx.doi.org/10.1371/journal.pmed.004032910.1371/journal.pmed.004032918092884 [Google Scholar] [CrossRef] [PubMed]
[31]. Cheng Y, Chen Z, Gawthorne JA, Mukerjee C, Varettas K, Mansfield KJ, Detection of intracellular bacteria in exfoliated urothelial cells from women with urge incontinencePathog Dis [Internet] 2016 74(7):ftw067Available from: https://dx.doi.org/10.1093/femspd/ftw06710.1093/femspd/ftw06727402784 [Google Scholar] [CrossRef] [PubMed]
[32]. Robino L, Scavone P, Araujo L, Algorta G, Zunino P, Pírez MC, Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in childrenClin Infect Dis [Internet] 2014 59(11):e158-64.Available from: https://dx.doi.org/10.1093/cid/ciu63410.1093/cid/ciu63425091303 [Google Scholar] [CrossRef] [PubMed]
[33]. Kim TG, Yi T, Lee EH, Ryu HW, Cho KS, Characterisation of a methane-oxidizing biofilm using microarray, and confocal microscopy with image and geostatic analysesAppl Microbiol Biotechnol [Internet] 2012 95(4):1051-59.Available from: https://dx.doi.org/10.1007/s00253-011-3728-y10.1007/s00253-011-3728-y22134640 [Google Scholar] [CrossRef] [PubMed]
[34]. Erokhina MV, Lepekha LN, Voronezhskaya EE, Nezlin LP, Avdienko VG, Ergeshov AE, Application of Laser scanning confocal microscopy for the visualization of M. Tuberculosis in lung tissue samples with weak Ziehl-Neelsen stainingJ Clin Med 2019 8(8):118510.3390/jcm808118531394889 [Google Scholar] [CrossRef] [PubMed]
[35]. Seiler P, Ulrichs T, Bandermann S, Pradl L, Jörg S, Krenn V, Cell-wall alterations as an attribute of Mycobacterium tuberculosis in latent infectionJ Infect Dis [Internet] 2003 188(9):1326-31.Available from: https://dx.doi.org/10.1086/37856310.1086/37856314593589 [Google Scholar] [CrossRef] [PubMed]
[36]. Zhang Y, Persistent and dormant tubercle bacilli and latent tuberculosisFront Biosci [Internet] 2004 9(1-3):1136-56.Available from: https://dx.doi.org/10.2741/129110.2741/129114977534 [Google Scholar] [CrossRef] [PubMed]
[37]. Kim JH, O’Brien KM, Sharma R, Boshoff HIM, Rehren G, Chakraborty S, A genetic strategy to identify targets for the development of drugs that prevent bacterial persistenceProc Natl Acad Sci U S A [Internet] 2013 110(47):19095-100.Available from: https://dx.doi.org/10.1073/pnas.131586011010.1073/pnas.131586011024191058 [Google Scholar] [CrossRef] [PubMed]
[38]. Naskalska A, Dabrowska A, Nowak P, Szczepanski A, Jasik K, Milewska A, Novel coronavirus-like particles targeting cells lining the respiratory tractPLoS One [Internet] 2018 13(9):e0203489Available from: https://dx.doi.org/10.1371/journal.pone.020348910.1371/journal.pone.020348930183777 [Google Scholar] [CrossRef] [PubMed]
[39]. Pulyaeva LV, Lukyanov KA, Studying SARS-CoV-2 with Fluorescence MicroscopyInt J Mol Sci 2021 22(12):655810.3390/ijms2212655834207305 [Google Scholar] [CrossRef] [PubMed]
[40]. Fellers TJ, Davidson MW, Introduction to confocal microscopyTallahassee, Florida [Google Scholar]
[41]. Pedergnana A, Ollé A, Evans AA, A new combined approach using confocal and scanning electron microscopy to image surface modifications on quartziteJ Archaeol Sci Rep [Internet] 2020 30(102237):102237Available from: https://dx.doi.org/10.1016/j.jasrep.2020.10223710.1016/j.jasrep.2020.102237 [Google Scholar] [CrossRef]
[42]. Borsari S, Pampena R, Lallas A, Kyrgidis A, Moscarella E, Benati E, Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosisJAMA Dermatol [Internet] 2016 152(10):1093-98.Available from: https://dx.doi.org/10.1001/jamadermatol.2016.118810.1001/jamadermatol.2016.118827580185 [Google Scholar] [CrossRef] [PubMed]
[43]. Ahlgrimm-Siess V, Laimer M, Rabinovitz HS, Confocal microscopy in skin cancerConfocal Microscopy in Skin Cancer Curr Derm Rep 2018 7:105-18.10.1007/s13671-018-0218-929780659 [Google Scholar] [CrossRef] [PubMed]
[44]. Ilie MA, Caruntu C, Lixandru D, Tampa M, Georgescu SR, Constantin MM, In-vivo confocal laser scanning microscopy imaging of skin inflammation: Clinical applications and research directionsExp Ther Med [Internet] 2019 17(2):1004-11.Available from: https://dx.doi.org/10.3892/etm.2018.698110.3892/etm.2018.6981 [Google Scholar] [CrossRef]
[45]. Pavlova I, Sokolov K, Drezek R, Malpica A, Follen M, Richards-Kortum R, Microanatomical and biochemical origins of normal and precancerous cervical autofluorescence using laser-scanning fluorescence confocal microscopyPhotochem Photobiol [Internet] 2003 77(5):550-55.Available from: https://dx.doi.org/10.1562/0031-8655(2003)0770550maboon2.0.co210.1562/0031-8655(2003)0770550MABOON2.0.CO2 [Google Scholar] [CrossRef]
[46]. Castellano-Muñoz M, Peng AW, Salles FT, Ricci AJ, Swept field laser confocal microscopy for enhanced spatial and temporal resolution in live-cell imagingMicrosc Microanal [Internet] 2012 18(4):753-60.Available from: https://dx.doi.org/10.1017/S143192761200054210.1017/S143192761200054222831554 [Google Scholar] [CrossRef] [PubMed]
[47]. Roberti MJ, Lopez LO, Ossato G, Steinmetz I, Haas P, Hecht F, TauSense: A fluorescence lifetime-based tool set for everyday imagingNat Methods 2020 [Google Scholar]
[48]. Alvarez LA, Widzgowski B, Ossato G, van den Broek B, Jalink K, Kuschel L, SP8 FALCON: A novel concept in fluorescence lifetime imaging enabling video-rate confocal FLIMNat Methods 2019 16(10) [Google Scholar]
[49]. Bayer C, Heindl NR, Rinke C, Lücker S, Ott JA, Bulgheresi S, Molecular characterisation of the symbionts associated with marine nematodes of the genus RobbeaEnvironmental Microbiology Reportss 2009 1(2):136-44.10.1111/j.1758-2229.2009.00019.x19838308 [Google Scholar] [CrossRef] [PubMed]