Introduction
The GDM is a common medical condition that occurs during pregnancy and is characterised by any degree of glucose intolerance first detected during pregnancy, but not clearly overt diabetes. It is usually diagnosed in the second or third trimester [1,2]. The global prevalence of GDM is 16%, with varying rates across countries. India has the highest prevalence at 29.3% [3]. Factors contributing to the development of GDM, similar to those for diabetes, include insulin resistance, β-cell dysfunction, gluconeogenesis, adipose tissue dysfunction, gut microbiota dysbiosis and oxidative stress [4].
Pathophysiology of GDM
Several factors, including genetic, environmental and nutritional influences, along with insulin resistance and β-cell dysfunction, may contribute to the development of GDM [5]. β-cell dysfunction can occur when β-cells fail to accurately sense blood glucose levels or produce enough insulin [6]. Pregnancy-induced insulin hyperplasia, which is caused by maternal obesity and placental hormones, can lead to insulin resistance. Pancreatic β-cells compensate for this by increasing insulin production; however, some women develop GDM due to an insufficient pancreatic response [7].
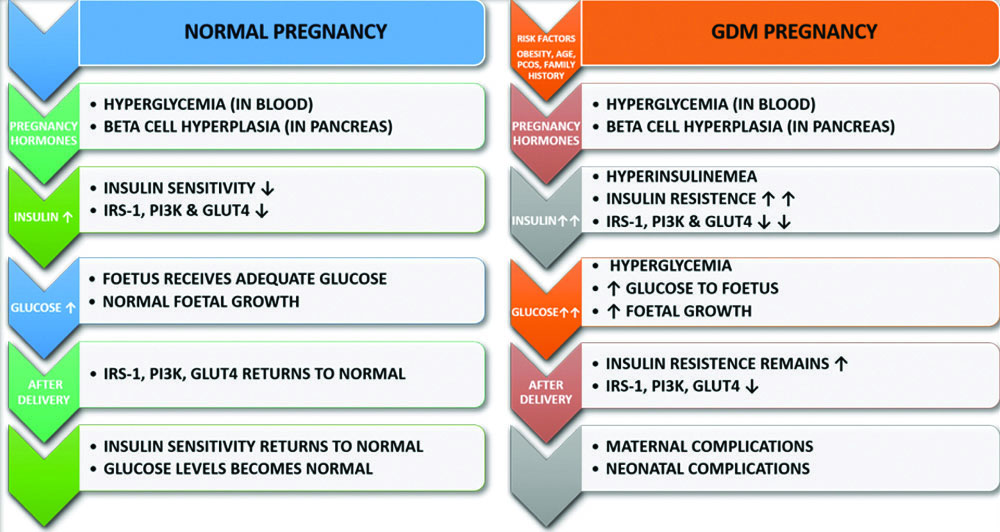
Insulin resistance is a condition in which cells fail to respond effectively to insulin because of impaired insulin signalling, resulting in inadequate translocation of Glucose Transporter-4 (GLUT4). GLUT4 is crucial for transporting glucose into cells for energy production. In GDM, insulin-stimulated glucose absorption is reduced by 54% compared to normal pregnancies. Insulin signalling is hindered by decreased tyrosine phosphorylation or increased serine/threonine phosphorylation of the insulin receptor. GDM is associated with altered expression or phosphorylation of downstream insulin signalling pathways, including GLUT4, Phosphoinositide-3-Kinase (PI3K) and Insulin Receptor Substrate (IRS)-1. Placental hormones play a crucial role in inducing insulin resistance [6,8]. [Table/Fig-1] illustrates the pathophysiology of normal and GDM pregnancies.
Pathophysiology of normal and GDM pregnancy.
↑ Increase, ↓ Decrease, PI3K-Phosphoinositide 3-Kinase, IRS-1-insulin Receptor Substrate-1 and GLUT 4-Glucose Transporter type 4
Current Diagnostic Modality
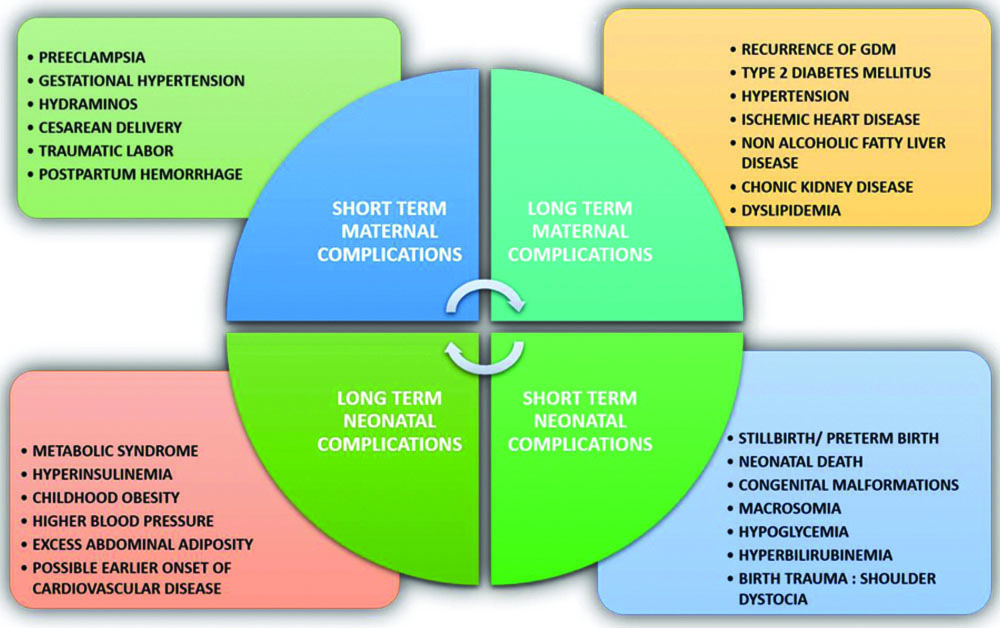
The OGTT is a standard diagnostic method for GDM in pregnant women, performed between 24 and 28 weeks of gestation. The International Association of Diabetes in Pregnancy Study Groups (IADPSG) criteria serve as the foundation for GDM diagnosis [9]. However, the lack of early screening methods, especially for low-risk populations, poses a challenge, as substantial foetal adiposity may have already developed by the time of diagnosis, contributing to maternal and neonatal complications, as shown in [Table/Fig-2] [5]. There is no universally accepted reference test for GDM diagnosis due to the varying diagnostic criteria used by different countries, as presented in [Table/Fig-3] [10-12]. The OGTT is considered the gold standard, but its reliability, speed and convenience raise concerns. The 75 g OGTT recommended by the IADPSG is significant but cumbersome due to its sampling requirements [13,14]. There is a pressing need for novel, accurate and timely diagnostic approaches for GDM. The present review emphasises the importance of exploring novel biomarkers from existing literature to enhance diagnostic methods.
Maternal and neonatal complications.
Diagnostic criteria for GDM [10-12].
| Guidelines | Glucose challenge | Fasting PG mg/dL (mmol/L) | 1-hour PG mg/dL (mmol/L) | 2-hour PG mg/dL (mmol/L) | 3-hour PG mg/dL (mmol/L) | Requirements |
|---|
| ACOG [10] | 100 g OGTT | ≥95 (5.3) | ≥180 (10.0) | ≥155 (8.6) | ≥140 (7.8) | Two or more values are required for diagnosis |
| WHO [11] | 75 g OGTT | ≥126 (7.0) | Not required | ≥140 (7.8) | Not required | One value sufficient for diagnosis |
| IADPSG [11] | 75 g OGTT | ≥92 (5.1) | ≥180 (10.0) | ≥153 (8.5) | Not required | One value sufficient for diagnosis |
| ADA [11] | 75 g OGTT | ≥95 (5.3) | ≥180 (10.0) | ≥155 (8.6) | Not required | Two or more values are required for diagnosis |
| DIPSI [12] | 75 g OGTT | Not required | Not required | ≥140 (7.8) | Not required | One value sufficient for diagnosis |
| CDA [12] | 75 g OGTT | ≥95 (5.3) | ≥191 (10.6) | ≥160 (8.9) | Not required | Two or more values are required for diagnosis |
PG: Plasma glucose; ACOG: American college of obstetricians and gynaecologists; WHO: World health organisation; IADPSG: International association of diabetes and pregnancy study groups; ADA: American diabetes association; DIPSI: Diabetes in pregnancy study group India; CDA: Canadian diabetes association
The biomarkers observed during the first and second trimesters of pregnancy, identified from the literature and their roles in GDM, are presented. Among these biomarkers, some are novel discoveries, while others may have been previously known but studied in contexts other than GDM.
Vitamin D
Vitamin D, a fat-soluble secosteroid also known as calciferol, is synthesised in the liver and kidneys through hydroxylation after exposure to sunlight [15]. It affects calcium channels and tissues such as the gut, vascular smooth muscle, pancreatic β-cells and monocytes. Vitamin D deficiency is linked to decreased levels of collagen type I, osteoblast activity, parathyroid hormone secretion, muscle function and insulin secretion [16]. Cholecalciferol increases Ca2+ influx, impacting insulin production in β-cells and leading to insulin exocytosis. Vitamin D deficiency is also associated with impaired glucose tolerance, primary hyperparathyroidism and Type 2 Diabetes Mellitus (T2DM) [17].
Soheilykhah S et al., suggest that vitamin D deficiency may elevate the risk of GDM due to its effects on insulin production, activity and sensitivity, potentially influencing β-cell function and secretion [18]. Amrein K et al., recommend that pregnant women with vitamin D levels below 40 ng/mL should be supplemented with 400-600 IU of vitamin D daily to prevent adverse outcomes [19]. A strong relationship has been observed between low vitamin D levels and the risk of GDM, with individuals with vitamin D deficiency having a 26% greater chance of developing GDM [20-22]. A randomised controlled trial found that calcium-based vitamin D supplementation reduced caesarean sections, maternal hospitalisations, macrosomia, hyperbilirubinemia and neonatal hospitalisations in women with GDM [23]. Zhang Q et al., found that administering 50,000 IU of vitamin D every two weeks decreased insulin resistance without affecting inflammation or triglyceride levels [24].
Previous research emphasises that prenatal vitamin D intake is crucial and routine screening can help assess the risk of GDM in individuals. In contrast to GDM, more research has been conducted on vitamin D, yet its exact mechanism behind GDM is not fully understood.
Vitamin B9
Vitamin B9, commonly known as folate, is a group of water-soluble vitamins essential for cell division and one-carbon metabolism. It is used in food fortification and supplements, with 5-methyltetrahydrofolate being the primary type found in plasma. Elevated folate levels can affect β-cell function and promote insulin differentiation in pig pancreatic stem cells. However, excessive doses can decrease cell growth and survival [25,26].
Chen X et al., revealed that pregnancies complicated by GDM have higher levels of folate and vitamin B12 and supplementation with folic acid increases the risk of GDM [27]. Studies have found a robust correlation between early pregnancy levels of vitamin B12 and folate [28,29]. Research on vitamin B9, including animal studies supporting its role in β-cell function, is limited [27,28], indicating a need for further human studies on its role in the pathophysiology of GDM.
Vitamin B12
Vitamin B12, also known as cobalamin, is crucial for physiological processes like cell division, DNA synthesis and amino acid metabolism, particularly during pregnancy [30]. It plays a vital role in the remethylation of homocysteine to methionine and the mitochondrial conversion of methylmalonyl-CoA to succinyl-CoA for energy production [31]. A deficiency in B12 can lead to functional folate deficiency and impaired DNA synthesis, potentially contributing to insulin resistance. Women with GDM have reduced mitochondrial activity, which is linked to increased methylation of the D-loop region of the mitochondrial genome [32]. Vitamin B12 deficiency can negatively affect DNA and cell metabolism, leading to reduced enzyme activity and impacting liver coenzyme A and glucose breakdown. Monitoring vitamin B12 levels is essential, especially in individuals with T2DM, as diminished levels can impair the maintenance of enzyme systems [33].
Wang L et al., revealed a connection between GDM and lower serum concentrations of vitamin B12 [34]. Low levels during the second or third trimester increase the risk, especially among Asians, while adequate vitamin B12 status reduces the risk of GDM. However, higher maternal blood levels in early pregnancy may elevate this risk [35]. Kanwal A et al., unveiled significant variations in vitamin B12 levels, with low glutathione peroxidase and high homocysteine levels associated with GDM [36].
Preptin
Preptin, an E-peptide derived from proinsulin-like growth factor II, is co-secreted with insulin in islet β-cell granules in response to glucose, enhancing glucose-mediated insulin production. Individuals with metabolic disorders such as GDM, Polycystic Ovarian Syndrome (PCOS), T2DM and impaired glucose tolerance often have elevated preptin levels. A study by Cheng KC et al., indicated that preptin influences insulin secretion by activating the Protein Kinase C/Phospholipase C (PKC/PLC) pathway and affecting signal transduction through the Insulin-like Growth Factor 2 Receptor (IGF2R) [37]. Additionally, a 2019 study suggested that high preptin levels in insulin-resistant patients may indicate a link between preptin and glucose-lipid metabolism, potentially contributing to insulin resistance [38].
Research by Kirac UI et al., found that individuals with elevated serum preptin levels have a higher risk of developing GDM [39]. However, another study found that maternal serum preptin levels were comparable to those of non GDM control subjects, while cord blood preptin levels were lower [40]. Preptin appears to be a novel hormone. Despite limited research, studies suggest that preptin is related to insulin resistance and is synthesised in response to glucose. Further research is warranted to elucidate its role in the pathogenesis of GDM.
Angiopoietin Like Protein 8
Angiopoietin-like 8 (ANGPTL8) is a 22-kDa peptide primarily synthesised in the liver and adipose tissues of humans. It plays a crucial role in lipid and glucose metabolism by enhancing insulin sensitivity, promoting glycogen production and improving glucose tolerance [41]. Additionally, ANGPTL8 regulates lipid metabolism by inhibiting the action of lipoprotein lipase, thereby increasing triglyceride levels and reducing serum-free fatty acid levels. It is also known by several alternative names, such as betatrophin, Refeeding Induced Fat and Liver (RIFL) protein and lipasin [42].
Furthermore, ANGPTL8 decreases fasting blood glucose levels and enhances glucose tolerance. Overexpression of ANGPTL8 inhibits gluconeogenesis, suggesting a role in suppressing hepatic gluconeogenesis [43]. Seyhanli Z et al., found a significant correlation between ANGPTL8 levels and insulin resistance, suggesting ANGPTL8’s potential as a biomarker for GDM [44].
Adiponectin
Adiponectin, an anti-inflammatory adipokine, plays a crucial role in lipid and glucose metabolism, inflammation, cell apoptosis and angiogenesis. In women with GDM, lower maternal levels of adiponectin are associated with higher birth weights, potentially contributing to insulin resistance and foetal overgrowth [45]. Studies in rodents have demonstrated that full-length adiponectin inhibits foetal growth, while its absence results in enlargement [46]. Adiponectin interacts with receptors AdipoR1 and AdipoR2, activating signalling pathways like AMP-activated Protein Kinase (AMPK) and Peroxisome Proliferator-activated Receptor (PPAR) α. This interaction facilitates GLUT4 translocation, leading to cellular responses, including glucose suppression, lipogenesis, inflammation modulation, glucose uptake, fatty acid oxidation and enhanced insulin sensitivity [47].
Additionally, adiponectin suppresses the production of amino acids and nutrient transporters like GLUT-1 in human villous cytotrophoblasts. Studies have shown that decreased adiponectin levels in the placentas of women with GDM, coupled with increased maternal Insulin-like Growth Factor (IGF-1), activate insulin/IGF-1 signalling pathways, potentially impacting foetal development [46]. Early in pregnancy, women diagnosed with GDM exhibit lower levels of adiponectin, which are correlated with visceral adiposity and glucose control [48].
Chemerin
Chemerin, classified as a novel adipokine, plays a crucial role in regulating glucose and fat metabolism. It originates from the retinoic acid receptor responder 2 gene and initially exists as a 163-amino acid inactive preproprotein [45]. Chemerin functions by modulating proinflammatory cytokines and contributing to adipocyte differentiation. Impairment of chemerin receptors can lead to diminished glucose tolerance, obesity and reduced levels of proinflammatory cytokines in adipose tissue [49].
Wang X et al., have highlighted that chemerin levels are positively correlated with inflammatory markers like TNF-alpha and IL-6. Women with GDM have higher plasma chemerin levels compared to normal pregnant women [50]. Studies have shown that mutations affecting rs4721 (Beta-2 adrenergic-receptor gene) and rs17173608 (Fat-mass and obesity-associated gene) polymorphisms in the Chinese population are associated with decreased plasma chemerin levels, a low Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) score and protection against the development of GDM [51]. A case-control study found higher chemerin messenger Ribonucleic Acid (mRNA) and protein expression in the placenta, adipose tissue and umbilical cord blood in women with GDM compared to the normal group [52].
Omentin-1
Omentin-1, an adipokine, is crucial for controlling microbiota across various organs and possesses anti-inflammatory properties. Impaired production of omentin-1 is linked to coronary artery disease, insulin resistance and diabetes. It enhances insulin sensitivity and facilitates glucose absorption, ensuring adequate levels for maintaining insulin sensitivity and metabolic balance [49].
Interventions targeting weight loss in individuals with T2DM and obesity have been shown to elevate omentin-1 levels [53].
Franz M et al., found that omentin-1 levels decrease during pregnancy, with women of higher Body Mass Index (BMI) showing lower levels. Offspring of diabetic women also have lower omentin-1 levels. The study suggests that altered insulin sensitivity during pregnancy could lead to decreased levels in the third trimester [54]. Overall, omentin-1 appears to be more closely associated with BMI and obesity than directly with insulin and glucose homeostasis.
Leptin
Leptin, an adipokine, is closely associated with insulin resistance and obesity. It regulates food intake and correlates with adipocyte mass. During pregnancy, leptin levels peak around the 28th week of gestation and women diagnosed with GDM show increased levels of circulating leptin and higher leptin messenger RNA expression [55]. Leptin regulates glucose homeostasis and increases during pregnancy due to changes in maternal fat stores and glucose metabolism. It directly impacts whole-body insulin sensitivity by regulating gluconeogenesis in skeletal muscle and the liver. Studies suggest that leptin acutely inhibits insulin secretion [56]. Xiao WQ et al., highlighted that women with GDM have higher concentrations of leptin compared to those without GDM and circulating leptin levels are notably elevated in women with GDM compared to normal pregnant women [57].
Interleukin-6 (IL-6)
The IL-6 an inflammatory cytokine, is released by various cell types and plays a crucial role in regulating inflammation, insulin resistance, the immune system, obesity and type 2 diabetes. It may contribute to GDM by promoting adipose tissue expansion, inflammation, or release from the placenta [45].
Women with GDM typically have elevated levels of IL-6 compared to those of normal pregnant women. During pregnancy, IL-6 secretion by the placenta may increase in response to hyperleptinemia. Research suggests that IL-6 could serve as a valuable biomarker for early glucose intolerance screening. GDM can induce insulin resistance due to heightened IL-6 levels and leptin stimulates monocytes via Mitogen-Activated Protein Kinase/Extracellular Signal-regulated Kinase (MAPK/ERK) signalling pathways, enhancing IL-6 production [58]. Amirian A et al., found a significant association between IL-6 levels and GDM, suggesting that assessing serum IL-6 levels could serve as a new diagnostic biomarker for GDM [59].
Homocysteine
Homocysteine, a sulfur-containing amino acid produced from the hydrolysis of S-adenosylhomocysteine, plays a crucial role in one-carbon metabolism within the methionine cycle and is linked to various pathological conditions, including diabetic vascular disease, insulin resistance, thrombotic events and premature arterial atherosclerotic disease [60]. Homocysteine levels are typically lower during normal pregnancies; however, hyperhomocysteinemia can lead to pregnancy complications such as recurrent miscarriages, hypertension, preterm delivery, GDM, placental abruption and foetal growth restriction [61].
Zhang X et al., suggest that elevated homocysteine levels decrease the insulin receptor alpha and beta subunits, thereby affecting insulin sensitivity. Treatment with homocysteine reduces the protein levels of these subunits, contributing to insulin resistance and diabetes. The cysteine-homocysteinylation mechanism of the pro-insulin receptor is proposed as the underlying mechanism. Inhibiting cysteine-homocysteine interactions in the endoplasmic reticulum may improve insulin sensitivity and help prevent insulin resistance [62]. Studies have found higher homocysteine levels in women with GDM [63,64]. Additionally, vitamin B12 insufficiency is linked to increased homocysteine levels and lower glutathione peroxidase levels [36].
C-Peptide
The short polypeptide known as the connecting peptide (C-peptide), which consists of 31 amino acids, is responsible for linking the A and B chains of proinsulin. It has a half-life of 20-30 minutes, making it more stable than insulin [65]. C-peptide’s physiological functions include acting as a structural bridge, stimulating Sodium–potassium Adenosine triphosphatase (Na+-K+-ATPase), modifying endothelial nitric oxide synthase and inducible nitric oxide synthase, influencing intracellular signalling, gene transcription, glucose metabolism, thrombotic events and lipid metabolism [66]. Beyond its structural roles, it also exhibits biological activity by attaching to G-protein-dependent receptors on cell membranes, modulating gene transcription and nuclear processes and demonstrating insulin-mimetic properties that regulate hyperglycaemia and glucose metabolism in diabetes [65].
Yang X et al., found a correlation between GDM and early pregnancy serum C-peptide levels, suggesting that C-peptide could be a reliable biomarker for predicting GDM risk in early pregnancy [67]. While the functional properties of C-peptide are not fully understood, its role as an indicator of GDM in early pregnancy warrants further investigation.
Irisin
Irisin, a neurokine, myokine and adipokine produced by the fibronectin type III domain containing-5 gene, regulates energy consumption and glucose metabolism by converting white adipose tissue into brown adipose tissue [68]. It acts as an anti-diabetic hormone by decreasing insulin resistance, increasing insulin sensitivity, reducing body weight, improving glucose tolerance, enhancing lipolysis, promoting the browning of white adipocytes, increasing GLUT4 expression and stimulating β-cell regeneration. Irisin secretion is upregulated by exercise and downregulated by hyperglycaemia and high fatty acid concentrations [69]. Irisin is implicated in metabolic disorders such as obesity, lipid metabolism disorders, cardiovascular disease, polycystic ovarian syndrome, T2DM and non alcoholic fatty liver disease [70]. Studies show that irisin levels are significantly lower in women with GDM compared to normal pregnant women, possibly due to insulin resistance or high oestrogen levels [40,49]. Further research is needed to fully understand irisin’s role in GDM during early pregnancy.
Adropin
Adropin, a regulatory protein present in the pancreas, liver, brain, kidneys and umbilical vein, is involved in preventing insulin resistance and obesity, as well as maintaining glucose and lipid homeostasis. Elevated levels of adropin have been observed in patients with T2DM, suggesting a potential role in the pathophysiology of GDM [71]. A meta-analysis revealed increased maternal serum adropin levels in patients with GDM compared to a control group. These elevated levels were particularly notable during the first and last trimesters of pregnancy [72]. A recent study found that patients with first-degree obesity in the third trimester had higher levels of adropin compared to those with third-degree obesity, suggesting that a lower Body Mass Index (BMI) may lead to endogenous adropin production, which protects against insulin resistance and hyperglycaemia [73].
Nesfatin-1
Nesfatin-1, an 82-amino acid peptide derived from nucleobindin-2, is secreted by peripheral organs and tissues and regulates hunger, satiety and body weight. It is primarily expressed in the hypothalamus and influences blood glucose levels in patients with diabetes [74]. Nesfatin-1 plays a crucial role in carbohydrate metabolism by enhancing glucose-induced insulin release, stimulating pre-proinsulin mRNA expression and inhibiting glucagon secretion. It is localised in pancreatic β-islets in mice and rats and has an antihyperglycemic effect when administered to hyperglycemic animals [71]. A study found that low maternal serum Nesfatin-1 levels were associated with a higher risk of developing GDM, with a 6.1 times higher likelihood of developing GDM in women with GDM compared to healthy pregnant women [75].
Afamin
Afamin, a liver-expressed albumin protein, is associated with the development of T2DM. It also contributes to oxidative stress and insulin resistance, which increase during pregnancy and are strongly linked to GDM [76]. Tramontana A et al., highlighted that women with GDM have higher median afamin concentrations, which serve as a robust predictor for GDM [77]. Similarly, Li Q et al., revealed that elevated afamin levels show higher sensitivity and specificity for GDM diagnosis and are positively correlated with HOMA-IR values and insulin levels. These findings suggest a potential role for afamin in pregnancy-related metabolic disorders [78].
Fetuin-A
Fetuin A, a member of the cystatin fetuin family and also known as a glycoprotein, is a naturally occurring inhibitor of the insulin receptor tyrosine kinase. It is released by adipose tissue and the liver. Fetuin A is linked to atherogenic lipid profiles, T2DM, insulin resistance, fat accumulation and metabolic syndrome [79]. Plasma concentrations of fetuin A are significantly increased in women with GDM compared to those with Normal Glucose Tolerance (NGT) controls, with levels rising from the first to the second trimester [80]. Jin C et al., found that women with GDM had higher plasma fetuin A concentrations, which are associated with changes in insulin resistance, fasting insulin levels and β-cell activity [81]. This suggests that fetuin A could serve as a biomarker for predicting the risk of GDM. The highest concentrations were linked to an increased risk of developing GDM.
Amylin
Amylin, a hormone released by pancreatic β-cells, plays a significant role in suppressing glucagon release, regulating glucose metabolism and controlling stomach emptying. It is predominantly found in the islets of Langerhans in over 90% of patients with T2DM and constitutes the primary component of amyloid. The development of T2DM is attributed to amylin fibrils inducing β-cell cytotoxicity, which leads to intracellular ion imbalances, membrane damage and β-cell death [71]. However, more studies are needed to validate the potential relationship between amylin and the development of GDM due to insufficient data.
Galectin
Galectins are a diverse family of lectins with broad specificity, involved in cell-matrix adhesion, cell-cell interactions and transmembrane signalling. They play roles in angiogenesis, maternal immune responses and placentation during pregnancy. A total 10 of the 16 classes of galectins are found in humans, predominantly in placental tissue. Galectin-1 and -3 are decreased in the placentas of patients with GDM, while the role of galectin-2 remains uncertain [49]. Pelech A et al., found that the immunomodulatory protein galectin-9 binds to GLUT-2, an essential component of pancreatic β-cells and regulates glucose homeostasis [82]. Research by Buschmann C et al., indicates that, compared to the healthy control group, placentas affected by GDM exhibit overexpression of galectin-12 in the nucleus of the syncytiotrophoblast and the extravillous trophoblast [83]. Furthermore, investigations are needed to elucidate the specific role of galectin-12 in the placenta and its association with GDM.
Osteocalcin
Osteocalcin, a non collagenous protein found in bone, plays a role in regulating fat and glucose metabolism. It indirectly lowers blood glucose levels by promoting the utilisation of glucose by the liver, adipocytes and muscle tissues, or by increasing insulin release from pancreatic β-cells [84]. A meta-analysis revealed increased levels of osteocalcin in women with GDM compared to normal pregnant women [85]. Osteocalcin exists in three main forms: undercarboxylated Osteocalcin (ucOC), total Osteocalcin (tOC) and carboxylated osteocalcin (cOC). While pregnant individuals with and without GDM showed no significant changes in serum tOC concentrations, women with GDM exhibited significantly elevated serum levels of ucOC [86].
Resistin
Resistin, a proinflammatory adipokine produced in various cells, has been shown to inhibit insulin’s effects in mice. It is associated with metabolic syndrome, inflammation, diabetes and obesity, contributing to the pathophysiology of GDM by reducing insulin sensitivity, elevating plasma glucose concentrations and inhibiting glucose absorption [49]. Bawah AT et al., conducted a study during the first trimester of pregnancy and found that women with GDM had a significant increase in serum resistin levels compared to non GDM women [87]. Additionally, a meta-analysis further supports the link between maternal serum resistin levels and the risk of GDM [88].
Visfatin
Visfatin, primarily produced by visceral adipose tissue in both mice and humans, is also known as a pre-B cell colony-enhancing factor and nicotinamide phosphoribosyltransferase. It plays a significant role in energy homeostasis and the development of GDM and is expressed in various tissues [89]. Prior studies have revealed that patients with GDM exhibit higher levels of visfatin, particularly during the first trimester of pregnancy [90]. Furthermore, a similar survey by Bawah AT et al., supports these findings [87]. These results suggest that elevated visfatin levels during gestation could serve as a novel biomarker for the early detection of GDM.
Fatty Acid-binding Protein 4
Fatty Acid-Binding Protein 4 (FABP4) is a lipid-binding protein found in adipose tissue and mature adipocytes. It enhances fatty acid transport and regulates lipid metabolism. FABP4 is associated with obesity, insulin resistance and T2DM, with higher levels observed in GDM [91]. A study by Ron I et al., found FABP4 expression in the white adipose tissues of pregnant women with GDM and those who were normoglycemic, with increased secretion from visceral white adipose tissues in GDM patients [92]. Neutralising FABP4 reduced glucagon-stimulated hepatic glucose production [85,92].
The biomarkers observed during the first and second trimesters of pregnancy, identified from the literature and their roles in GDM are presented in [Table/Fig-4] [17,26,32,38,43,47,49,51,53,56,58,62,67,69,71,73,76,79,82,84,89,92]. The present review provides an overview of GDM, focusing on its pathophysiology, complications, diagnosis and prediction. It emphasises the importance of early diagnosis and highlights the significance of existing biomarkers in the prediction and diagnosis of GDM. The present review will aid researchers in the initial stages of their literature review by outlining existing biomarkers for diagnosing and predicting GDM.
Biomarkers and their pathophysiology in gestational diabetes mellitus [17,26,32,38,43,47,49,51,53,56,58,62,67,69,71,73,76,79,82,84,89,92].
| Biomarkers | Levels in GDM | Pathophysiology |
|---|
| Vitamin D | ↓ | Insulin secretion-increases Ca2+ influx, impacting insulin production in β-cells [17] |
| Vitamin B9 | ↑ | Insulin production-influences β-cell function, promoting insulin differentiation in pancreatic cells [26] |
| Vitamin B12 | ↓ | Insulin resistance-the conversion of methyl malonyl-CoA to succinyl-CoA, which has been associated with insulin resistance [32] |
| Preptin | ↑ | Insulin secretion-enhancing glucose-mediated insulin production and influences insulin secretion by activating Protein Kinase C/Phospholipase C (PKC/PLC) pathway [38] |
| Angiopoietin-like protein 8 | ↑ | Enhancing insulin sensitivity, promoting glycogen production and improving glucose tolerance-inhibits gluconeogenesis through AKT signalling, suggesting a role in suppressing hepatic gluconeogenesis [43] |
| Adiponectin | ↓ | Insulin sensitivity-enhances AMPK signalling in the liver and skeletal muscle, promoting glucose uptake via GLUT4 translocation [47] |
| Chemerin | ↑ | Insulin resistance [51] |
| Omentin-1 | ↓ | Insulin sensitivity and glucose homeostasis [53] |
| Leptin | ↑ | Insulin sensitivity and hepatic regulation of gluconeogenesis [56] |
| Interleukin-6 | ↑ | Insulin resistance [58] |
| Homocysteine | ↑ | Insulin resistance caused by inhibiting pro-insulin receptor cleavage [62] |
| C-Peptide | ↑ | Indicator of GDM [67] |
| Irisin | ↓ | Insulin resistance, insulin sensitivity [69] |
| Adropin | ↑ | Insulin resistance, obesity [73] |
| Nesfatin-1 | ↓ | Enhancing glucose-induced insulin release, stimulating pre-proinsulin mRNA expression [71] |
| Afamin | ↑ | Insulin resistance [76] |
| Fetuin-A | ↑ | Inhibit insulin receptor tyrosine kinase activity, leading to insulin resistance [79] |
| Amylin | ↑ | Beta cell death [71] |
| Galectin | ↑ | Regulates glucose homeostasis [82] |
| Osteocalcin | ↑ | Increasing insulin release from pancreatic beta cells [84] |
| Resistin | ↑ | Insulin sensitivity, inhibiting glucose absorption [49] |
| Visfatin | ↑ | Energy homeostasis and development of GDM [89] |
| FABP4 | ↑ | Insulin resistance and obesity [92] |
↑: Increased Levels, ↓: Decreased Levels
Conclusion(s)
Among the biomarkers evaluated, preptin, homocysteine, C-peptide and vitamin D appear to be promising indicators for predicting the risk of GDM and for identifying high-risk individuals due to their strong association with the pathophysiology of GDM. Vitamin D has been studied extensively, but its exact mechanism remains unclear. Preptin and homocysteine are linked to insulin secretion and signalling pathways. However, the number of studies on these biomarkers is limited, necessitating larger sample size studies, meta-analyses and clinical trials. These biomarkers show potential for predicting GDM risk and identifying high-risk individuals, but further research is needed to explore additional adipokines and biomarkers.
The review underscores the importance of early pregnancy stages, particularly between 8 and 15 weeks, to improve the accuracy and timeliness of GDM detection. It suggests that microRNAs, which are being studied for various diseases, could help uncover the pathogenesis and mechanisms of GDM. However, the review primarily focuses on inflammation and protein biomarkers, neglecting genetic markers and their diverse physiological functions, which represents a limitation.
[1]. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancyDiabetes Care 2010 33(3):676-82.10.2337/dc09-184820190296 [Google Scholar] [CrossRef] [PubMed]
[2]. Dalfra MG, Burlina S, Del Vescovo GG, Lapolla A, Genetics and epigenetics: New insight on gestational diabetes mellitusFront Endocrinol 2020 11:60247710.3389/fendo.2020.60247733335512 [Google Scholar] [CrossRef] [PubMed]
[3]. Internet Source: International Diabetes Federation 2024 Jan 1 [cited 2024 Apr 28]. Available from: https://diabetesatlas.org/data/en/indicators/14/ [Google Scholar]
[4]. Lu W, Hu C, Molecular biomarkers for gestational diabetes mellitus and postpartum diabetesChin Med J (Engl) 2022 135(16):1940-51.10.1097/CM9.000000000000216036148588 [Google Scholar] [CrossRef] [PubMed]
[5]. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P, Gestational diabetes mellitusNat Rev Dis Primer 2019 5(1):4710.1038/s41572-019-0098-831296866 [Google Scholar] [CrossRef] [PubMed]
[6]. Plows J, Stanley J, Baker P, Reynolds C, Vickers M, The pathophysiology of gestational diabetes mellitusInt J Mol Sci 2018 19(11):334210.3390/ijms1911334230373146 [Google Scholar] [CrossRef] [PubMed]
[7]. Choudhury AA, Devi Rajeswari V, Gestational diabetes mellitus- A metabolic and reproductive disorderBiomed Pharmacother 2021 143:11218310.1016/j.biopha.2021.11218334560536 [Google Scholar] [CrossRef] [PubMed]
[8]. Alesi S, Ghelani D, Rassie K, Mousa A, Metabolomic biomarkers in gestational diabetes mellitus: A review of the evidenceInt J Mol Sci 2021 22(11):551210.3390/ijms2211551234073737 [Google Scholar] [CrossRef] [PubMed]
[9]. Lachmann EH, Fox RA, Dennison RA, Usher-Smith JA, Meek CL, Aiken CE, Barriers to completing oral glucose tolerance testing in women at risk of gestational diabetesDiabet Med 2020 37(9):1482-89.10.1111/dme.1429232144795 [Google Scholar] [CrossRef] [PubMed]
[10]. Internet Source: Updated ACOG Guidance on Gestational DiabetesThe ObG Project 2023 [cited 2024 Aug 10]. Available from: https://www.obgproject.com/2023/01/02/acog-releases-updated-guidancegestational-diabetes/ [Google Scholar]
[11]. Rani PR, Begum J, Screening and diagnosis of gestational diabetes mellitus, where do we standJ Clin Diagn Res 2016 10(4):QE01-QE04.10.7860/JCDR/2016/17588.768927190902 [Google Scholar] [CrossRef] [PubMed]
[12]. Nayak PK, Mitra S, Sahoo JP, Daniel M, Mathew A, Padma A, Feto-maternal outcomes in women with and without gestational diabetes mellitus according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteriaDiabetes Metab Syndr 2013 7(4):206-09.10.1016/j.dsx.2013.10.01724290085 [Google Scholar] [CrossRef] [PubMed]
[13]. McIntyre HD, Kapur A, Divakar H, Hod M, Gestational diabetes mellitus-innovative approach to prediction, diagnosis, management, and prevention of future NCD-mother and offspringFront Endocrinol 2020 11:61453310.3389/fendo.2020.61453333343512 [Google Scholar] [CrossRef] [PubMed]
[14]. Shen L, Zhao D, Chen Y, Zhang K, Chen X, Lin J, Comparative proteomics analysis of serum proteins in gestational diabetes during early and middle stages of pregnancyProteomics Clin Appl 2019 13(5):e180006010.1002/prca.20180006031162828 [Google Scholar] [CrossRef] [PubMed]
[15]. Keller A, Varela Vazquez C, Dangol R, Damm P, Heitmann BL, Händel MN, The role of vitamin d in the development of diabetes post gestational diabetes mellitus: A systematic literature reviewNutrients 2020 12(6):173310.3390/nu1206173332531957 [Google Scholar] [CrossRef] [PubMed]
[16]. Lips P, Vitamin D physiologyProg Biophys Mol Biol 2006 92(1):04-08.10.1016/j.pbiomolbio.2006.02.01616563471 [Google Scholar] [CrossRef] [PubMed]
[17]. Vondra K, Hampl R, Vitamin D and new insights into pathophysiology of type 2 diabetesHorm Mol Biol Clin Investig 2021 42(2):203-08.10.1515/hmbci-2020-005533655734 [Google Scholar] [CrossRef] [PubMed]
[18]. Soheilykhah S, Mojibian M, Rashidi M, Rahimi-Saghand S, Jafari F, Maternal Vitamin D status in gestational diabetes mellitusNutr Clin Pract 2010 25(5):524-27.10.1177/088453361037985120962313 [Google Scholar] [CrossRef] [PubMed]
[19]. Amrein K, Scherkl M, Hoffmann M, Neuwersch Sommeregger S, Köstenberger M, Tmava Berisha A, Vitamin D deficiency 2.0: An update on the current status worldwideEur J Clin Nutr 2020 74(11):1498-513.10.1038/s41430-020-0558-y31959942 [Google Scholar] [CrossRef] [PubMed]
[20]. Wang L, Zhang C, Song Y, Zhang Z, Serum vitamin D deficiency and risk of gestational diabetes mellitus: A meta-analysisArch Med Sci 2020 16(4):742-51.10.5114/aoms.2020.9443332542074 [Google Scholar] [CrossRef] [PubMed]
[21]. Milajerdi A, Abbasi F, Mousavi SM, Esmaillzadeh A, Maternal vitamin D status and risk of gestational diabetes mellitus: A systematic review and meta-analysis of prospective cohort studiesClin Nutr 2021 40(5):2576-86.10.1016/j.clnu.2021.03.03733933723 [Google Scholar] [CrossRef] [PubMed]
[22]. Cheng Y, Chen J, Li T, Pei J, Fan Y, He M, Maternal vitamin D status in early pregnancy and its association with gestational diabetes mellitus in Shanghai: A retrospective cohort studyBMC Pregnancy Childbirth 2022 22(1):81910.1186/s12884-022-05149-136335302 [Google Scholar] [CrossRef] [PubMed]
[23]. Karamali M, Asemi Z, Ahmadi-Dastjerdi M, Esmaillzadeh A, Calcium plus vitamin D supplementation affects pregnancy outcomes in gestational diabetes: Randomized, double-blind, placebo-controlled trialPublic Health Nutr 2016 19(1):156-63.10.1017/S136898001500060925790761 [Google Scholar] [CrossRef] [PubMed]
[24]. Zhang Q, Cheng Y, He M, Li T, Ma Z, Cheng H, Effect of various doses of vitamin D supplementation on pregnant women with gestational diabetes mellitus: A randomized controlled trialExp Ther Med 2016 12(3):1889-95.10.3892/etm.2016.351527588106 [Google Scholar] [CrossRef] [PubMed]
[25]. Naderi N, House JD, Recent developments in folate nutritionAdvances in Food and Nutrition Research 2018 83:195-213.10.1016/bs.afnr.2017.12.00629477222 [Google Scholar] [CrossRef] [PubMed]
[26]. Williamson JM, Arthurs AL, Smith MD, Roberts CT, Jankovic-Karasoulos T, High folate, perturbed one-carbon metabolism and gestational diabetes mellitusNutrients 2022 14(19):393010.3390/nu1419393036235580 [Google Scholar] [CrossRef] [PubMed]
[27]. Chen X, Zhang Y, Chen H, Jiang Y, Wang Y, Wang D, Association of maternal folate and Vitamin b12 in early pregnancy with gestational diabetes mellitus: A prospective cohort studyDiabetes Care 2021 44(1):217-23.10.2337/dc20-160733158950 [Google Scholar] [CrossRef] [PubMed]
[28]. Liu XH, Cao ZJ, Chen LW, Zhang DL, Qu XX, Li YH, The association between serum folate and gestational diabetes mellitus: A large retrospective cohort study in Chinese populationPublic Health Nutr 2023 26(5):1014-21.10.1017/S136898002200194X36093642 [Google Scholar] [CrossRef] [PubMed]
[29]. Yang Y, Cai Z, Zhang J, Association between maternal folate status and gestational diabetes mellitusFood Sci Nutr 2021 9(4):2042-52.10.1002/fsn3.217333841822 [Google Scholar] [CrossRef] [PubMed]
[30]. Chen X, Du Y, Xia S, Li Z, Liu J, Vitamin B 12 and gestational diabetes mellitus: A systematic review and meta-analysisBr J Nutr 2023 129(8):1324-31.10.1017/S000711452200246X35915058 [Google Scholar] [CrossRef] [PubMed]
[31]. Dib MJ, Gumban-Marasigan M, Yoxall R, Andrew T, Harrington DJ, Sobczyńska-Malefora A, Evaluating the diagnostic value of a combined indicator of Vitamin B12 Status (cB12) throughout pregnancyFront Nutr 2022 8:78935710.3389/fnut.2021.78935735155515 [Google Scholar] [CrossRef] [PubMed]
[32]. Maher A, Sobczyńska-Malefora A, The relationship between folate, Vitamin B12 and gestational diabetes mellitus with proposed mechanisms and foetal implicationsJ Fam Reprod Health 2021 15(3):141-49.10.18502/jfrh.v15i3.713134721605 [Google Scholar] [CrossRef] [PubMed]
[33]. Butola LK, Kute PK, Anjankar A, Dhok A, Gusain N, Vagga A, Vitamin B12- do you know everything?J Evol Med Dent Sci 2020 9(42):3139-46.10.14260/jemds/2020/688 [Google Scholar] [CrossRef]
[34]. Wang L, Hou Y, Meng D, Yang L, Meng X, Liu F, Vitamin B12 and folate levels during pregnancy and risk of gestational diabetes mellitus: A systematic review and meta-analysisFront Nutr 2021 8:67028910.3389/fnut.2021.67028934195216 [Google Scholar] [CrossRef] [PubMed]
[35]. Wang N, Zhou T, Ma X, Lin Y, Ding Y, The association between Maternal B Vitamins in early pregnancy and gestational diabetes mellitus: A prospective cohort studyNutrients 2022 14(23):501610.3390/nu1423501636501046 [Google Scholar] [CrossRef] [PubMed]
[36]. Kanwal A, Khalid A, Shahid A, Pasha HH, Rana M, Saeed MS, Vitamin B12, homocysteine and glutathione peroxidase levels in women with gestational diabetes mellitusGomal J Med Sci 2023 21(1):16-20.10.51253/pafmj.v73i1.7728 [Google Scholar] [CrossRef]
[37]. Cheng KC, Li YX, Asakawa A, Ushikai M, Kato I, Sato Y, Characterization of preptin-induced insulin secretion in pancreatic β-cellsJ Endocrinol 2012 215(1):43-49.10.1530/JOE-12-017622787110 [Google Scholar] [CrossRef] [PubMed]
[38]. Yoldemir SA, Altun O, The relationship between insulin resistance and serum preptin levelAnk Med J 2019 19(4):708-15.10.17098/amj.651958 [Google Scholar] [CrossRef]
[39]. Kirac UI, Demir E, Ozkan H, Sahtiyanci B, Uzun H, Ekinci I, Maternal serum preptin levels in the pathogenesis and diagnosis of Gestational diabetes mellitusJ Med Biochem 2023 42(2):311-17.10.5937/jomb0-3628736987416 [Google Scholar] [CrossRef] [PubMed]
[40]. Ersahin S, Yurci A, Cord blood and maternal serum preptin and irisin concentrations are regulated independently in GDMEur Rev Med Pharmacol Sci 2021 25:1954-58. [Google Scholar]
[41]. Yuan J, Zhang D, Wang Y, Zhu Z, Lin Q, Li M, Angiopoietin-like 8 in gestational diabetes mellitus: Reduced levels in third trimester maternal serum and placenta, increased levels in cord blood serum. Perez-Lopez FR, editorInt J Endocrinol 2022 2022:111381110.1155/2022/111381135529083 [Google Scholar] [CrossRef] [PubMed]
[42]. Guo C, Zhao Z, Deng X, Chen Z, Tu Z, Yuan G, Regulation of angiopoietin-like protein 8 expression under different nutritional and metabolic statusEndocr J 2019 66(12):1039-46.10.1507/endocrj.EJ19-026331631098 [Google Scholar] [CrossRef] [PubMed]
[43]. Zhao Z, Deng X, Jia J, Zhao L, Wang C, Cai Z, Angiopoietin-like protein 8 (betatrophin) inhibits hepatic gluconeogenesis through PI3K/Akt signalling pathway in diabetic miceMetabolism 2022 126:15492110.1016/j.metabol.2021.15492134715116 [Google Scholar] [CrossRef] [PubMed]
[44]. Seyhanli Z, Seyhanli A, Aksun S, Pamuk BO, Evaluation of serum Angiopoietin-like protein 2 (ANGPTL-2), Angiopoietin-like protein 8 (ANGPTL-8), and high-sensitivity C-reactive protein (hs-CRP) levels in patients with gestational diabetes mellitus and normoglycaemic pregnant womenJ Matern Fetal Neonatal Med 2022 35(25):5647-52.10.1080/14767058.2021.188891933615956 [Google Scholar] [CrossRef] [PubMed]
[45]. Bogdanet D, Reddin C, Murphy D, Doheny HC, Halperin JA, Dunne F, Emerging protein biomarkers for the diagnosis or prediction of gestational diabetes-a scoping reviewJ Clin Med 2021 10(7):153310.3390/jcm1007153333917484 [Google Scholar] [CrossRef] [PubMed]
[46]. Balachandiran M, Bobby Z, Dorairajan G, Gladwin V, Vinayagam V, Packirisamy RM, Decreased maternal serum adiponectin and increased insulin-like growth factor-1 levels along with increased placental glucose transporter-1 expression in gestational diabetes mellitus: Possible role in fetal overgrowthPlacenta 2021 104:71-80.10.1016/j.placenta.2020.11.00833285436 [Google Scholar] [CrossRef] [PubMed]
[47]. Pheiffer C, Dias S, Jack B, Malaza N, Adam S, Adiponectin as a potential biomarker for pregnancy disordersInt J Mol Sci 2021 22(3):132610.3390/ijms2203132633572712 [Google Scholar] [CrossRef] [PubMed]
[48]. Karasek D, Krystynik O, Kucerova V, Macakova D, Cibickova L, Schovanek J, Adiponectin, A-FABP and FGF-19 levels in women with early diagnosed gestational diabetesJ Clin Med 2022 11(9):241710.3390/jcm1109241735566542 [Google Scholar] [CrossRef] [PubMed]
[49]. Ruszała M, Niebrzydowska M, Pilszyk A, Kimber-Trojnar Ż, Trojnar M, Leszczyńska-Gorzelak B, Novel biomolecules in the pathogenesis of gestational diabetes mellitusInt J Mol Sci 2021 22(21):1157810.3390/ijms22211157834769010 [Google Scholar] [CrossRef] [PubMed]
[50]. Wang X, Liu J, Wang D, Zhu H, Kang L, Jiang J, Expression and correlation of Chemerin and FABP4 in peripheral blood of gestational diabetes mellitus patientsExperimental and Therapeutic Medicine 2020 19(1):710-16.10.3892/etm.2019.8247 [Google Scholar] [CrossRef]
[51]. Wang D, Wang H, Li M, Zhao R, Chemerin levels and its genetic variants are associated with Gestational diabetes mellitus: A hospital-based study in a Chinese cohortGene 2022 807:14588810.1016/j.gene.2021.14588834371096 [Google Scholar] [CrossRef] [PubMed]
[52]. Ma Z, Chu L, Zhang Y, Lu F, Zhu Y, Wu F, Is chemerin associated with gestational diabetes mellitus? A case-control studyDiabetes Metab Syndr Obes 2023 16:2271-81.10.2147/DMSO.S41763237551337 [Google Scholar] [CrossRef] [PubMed]
[53]. Cui S, Zhu X, Li S, Zhang C, Study on the predictive value of serum hypersensitive C-reactive protein, homocysteine, fibrinogen, and omentin-1 levels with gestational diabetes mellitusGynecol Endocrinol 2023 39(1):218304610.1080/09513590.2023.218304636996863 [Google Scholar] [CrossRef] [PubMed]
[54]. Franz M, Polterauer M, Springer S, Kuessel L, Haslinger P, Worda C, Maternal and neonatal omentin-1 levels in gestational diabetesArch Gynecol Obstet 2018 297(4):885-89.10.1007/s00404-018-4652-529335783 [Google Scholar] [CrossRef] [PubMed]
[55]. Rodrigo N, Glastras SJ, Pathophysiology underpinning gestational diabetes mellitus and the role of biomarkers for its predictionEMJ Diabetes 2020 [Google Scholar]
[56]. Soheilykhah S, Mojibian M, Rahimi-Saghand S, Rashidi M, Hadinedoushan H, Maternal serum leptin concentration in gestational diabetesTaiwan J Obstet Gynecol 2011 50(2):149-53.10.1016/j.tjog.2011.01.03421791299 [Google Scholar] [CrossRef] [PubMed]
[57]. Xiao WQ, He JR, Shen SY, Lu JH, Kuang YS, Wei XL, Maternal circulating leptin profile during pregnancy and gestational diabetes mellitusDiabetes Res Clin Pract 2020 161:10804110.1016/j.diabres.2020.10804132006645 [Google Scholar] [CrossRef] [PubMed]
[58]. Srivastava N, Singh K, Singh N, Mahdi AA, Association between serum interleukin-6, leptin and insulin in gestational diabetes mellitus-a cross- sectional studyJ Diabetes Metab Disord 2023 22(1):639-48.10.1007/s40200-023-01188-337255771 [Google Scholar] [CrossRef] [PubMed]
[59]. Amirian A, Mahani MB, Abdi F, Role of interleukin-6 (IL-6) in predicting gestational diabetes mellitusObstet Gynecol Sci 2020 63(4):407-16.10.5468/ogs.2002032689769 [Google Scholar] [CrossRef] [PubMed]
[60]. Zheng Y, Deng HY, Qiao ZY, Gong FX, Homocysteine level and gestational diabetes mellitus: A systematic review and meta-analysisGynecol Endocrinol 2021 37(11):987-94.10.1080/09513590.2021.196731434409893 [Google Scholar] [CrossRef] [PubMed]
[61]. Dai C, Fei Y, Li J, Shi Y, Yang X, A novel review of homocysteine and pregnancy complicationsBioMed Res Int 2021 2021:665223110.1155/2021/665223134036101 [Google Scholar] [CrossRef] [PubMed]
[62]. Zhang X, Qu YY, Liu L, Qiao YN, Geng HR, Lin Y, Homocysteine inhibits pro-insulin receptor cleavage and causes insulin resistance via protein cysteine-homocysteinylationCell Rep 2021 37(2):10982110.1016/j.celrep.2021.10982134644569 [Google Scholar] [CrossRef] [PubMed]
[63]. Deng M, Zhou J, Tang Z, Xiang J, Yi J, Peng Y, The correlation between plasma total homocysteine level and gestational diabetes mellitus in a Chinese Han populationSci Rep 2020 10(1):1867910.1038/s41598-020-75797-w33122744 [Google Scholar] [CrossRef] [PubMed]
[64]. Mahmood K, Al-Rasol EA, The effect of Vit B12 deficiency, homocystein, and lipid metabolism in association with increased risk of gestational diabetes mellitusMed J Babylon 2022 19(3):40910.4103/MJBL.MJBL_53_22 [Google Scholar] [CrossRef]
[65]. Yaribeygi H, Maleki M, Sathyapalan T, Sahebkar A, The effect of C-peptide on diabetic nephropathy: A review of molecular mechanismsLife Sci 2019 237:11695010.1016/j.lfs.2019.11695031605709 [Google Scholar] [CrossRef] [PubMed]
[66]. Wahren J, Ekberg K, Johansson J, Henriksson M, Pramanik A, Johansson BL, Role of C-peptide in human physiologyAm J Physiol-Endocrinol Metab 2000 278(5):E759-68.10.1152/ajpendo.2000.278.5.E75910780930 [Google Scholar] [CrossRef] [PubMed]
[67]. Yang X, Ye Y, Wang Y, Wu P, Lu Q, Liu Y, Association between early-pregnancy serum C-peptide and risk of gestational diabetes mellitus: A nested case-control study among Chinese womenNutr Metab 2022 19(1):5610.1186/s12986-022-00691-335996181 [Google Scholar] [CrossRef] [PubMed]
[68]. AL-Ghazali MJ, Ali HA, AL-Rufaie MM, Serum irisin levels as a potential marker for diagnosis of gestational diabetes mellitusActa Bio Medica Atenei Parm 2020 91(1):56-63. [Google Scholar]
[69]. Sahoo D, Pattanaik SR, Kumar PR, Gandhi R, Role of serum iris in during early pregnancy to predict the development of gestational diabetes mellitus at 24-28 weeks of pregnancy in high-risk patientsIndian J Endocrinol Metab 2022 26(1):61-67.10.4103/ijem.ijem_466_2135662754 [Google Scholar] [CrossRef] [PubMed]
[70]. Cui L, Qiao T, Xu F, Li Z, Chen T, Su H, Circulating irisin levels of prenatal and postnatal patients with gestational diabetes mellitus: A systematic review and meta-analysisCytokine 2020 126:15492410.1016/j.cyto.2019.15492431864100 [Google Scholar] [CrossRef] [PubMed]
[71]. Ruszala M, Pilszyk A, Niebrzydowska M, Kimber-Trojnar Ż, Trojnar M, Leszczyńska-Gorzelak B, Novel biomolecules in the pathogenesis of gestational diabetes mellitus 2.0Int J Mol Sci 2022 23(8):436410.3390/ijms2308436435457182 [Google Scholar] [CrossRef] [PubMed]
[72]. Vivek K, Reddy EP, Thangappazham B, Raj H, Pérez-López FR, Varikasuvu SR, Maternal adropin levels in patients with gestational diabetes mellitus: A systematic review and meta-analysisGynecol Endocrinol 2022 38(2):105-09.10.1080/09513590.2021.196370334378485 [Google Scholar] [CrossRef] [PubMed]
[73]. Adamczak L, Gutaj P, Wender-Ozegowska E, Adropin in pregnancy complicated by hyperglycemia and obesity-a preliminary studyGinekol Pol 2023 94(3):229-32.10.5603/GP.a2022.003135894495 [Google Scholar] [CrossRef] [PubMed]
[74]. Mierzynski R, Poniedzialek-Czajkowska E, Dluski D, Leszczyńska-Gorzelak B, The role of new adipokines in gestational diabetes mellitus pathogenesisGinekol Pol 2018 89(4):222-27.10.5603/GP.a2018.003829781079 [Google Scholar] [CrossRef] [PubMed]
[75]. Wahid UA, Islam MM, Ferdoues T, Saha S, Rizwan A, Association of maternal serum nesfatin-1 level with Gestational Diabetes Mellitus (GDM) in a tertiary care hospitalM Abdur Rahim Medical College Journal 2023 16(2):215-22. [Google Scholar]
[76]. Atakul N, Atamer Y, Selek Ş, Kılıç BS, Unal F, Novel metabolic marker Afamin: A predictive factor for Large-for-Gestational-Age (LGA) fetus estimation in pregnancies with gestational diabetes mellitus?J Gynecol Obstet Hum Reprod 2021 50(10):10220110.1016/j.jogoh.2021.10220134365029 [Google Scholar] [CrossRef] [PubMed]
[77]. Tramontana A, Pablik E, Stangl G, Hartmann B, Dieplinger H, Hafner E, Combination of first trimester serum afamin levels and three-dimensional placental bed vascularization as a possible screening method to detect women at-risk for adverse pregnancy complications like pre-eclampsia and gestational diabetes mellitus in low-risk pregnanciesPlacenta 2018 62:9-15.10.1016/j.placenta.2017.12.01429405972 [Google Scholar] [CrossRef] [PubMed]
[78]. Li Q, Li C, Jin J, Shen Y, Wang M, Clinical significance of Neuregulin 4, Afamin, and SERPINB1 in gestational diabetes mellitus and their relationship with insulin resistanceEvid Based Complement Alternat Med 2022 2022(9):01-08.10.1155/2022/282966236072413 [Google Scholar] [CrossRef] [PubMed]
[79]. Simjak P, Cinkajzlova A, Anderlova K, Klouckova J, Kratochvílová H, Lacinova Z, Changes in plasma concentrations and mRNA expression of Hepatokines Fetuin A, Fetuin B and FGF21 in physiological pregnancy and gestational diabetes mellitusPhysiol Res 2018 67(3):S531-42.10.33549/physiolres.93401730484680 [Google Scholar] [CrossRef] [PubMed]
[80]. Kansu-Celik H, Ozgu-Erdinc AS, Kisa B, Findik RB, Yilmaz C, Tasci Y, Prediction of gestational diabetes mellitus in the first trimester: Comparison of maternal fetuin-A, N-terminal proatrial natriuretic peptide, high-sensitivity C-reactive protein, and fasting glucose levelsArch Endocrinol Metab 2019 63(2):121-27.10.20945/2359-399700000012631038593 [Google Scholar] [CrossRef] [PubMed]
[81]. Jin C, Lin L, Han N, Zhao Z, Liu Z, Luo S, Effects of dynamic change in fetuin-A levels from the first to the second trimester on insulin resistance and gestational diabetes mellitus: A nested case-control studyBMJ Open Diabetes Res Care 2020 8(1):e00080210.1136/bmjdrc-2019-00080231958310 [Google Scholar] [CrossRef] [PubMed]
[82]. Pelech A, Ruszala M, Niebrzydowska-Tatus M, Bien K, Kimber-Trojnar Z, Czuba M, Do serum Galectin-9 levels in women with gestational diabetes and healthy ones differ before or after delivery? A pilot studyBiomolecules 2023 13(4):69710.3390/biom1304069737189444 [Google Scholar] [CrossRef] [PubMed]
[83]. Buschmann C, Unverdorben L, Knabl J, Hutter S, Meister S, Beyer S, Placental expression of inflammatory Galectin-12 is associated with gestational diabetesJ Reprod Immunol 2024 163:10424010.1016/j.jri.2024.10424038492532 [Google Scholar] [CrossRef] [PubMed]
[84]. Song L, Huang Y, Long J, Li Y, Pan Z, Fang F, The role of osteocalcin in placental function in gestational diabetes mellitusReprod Biol 2021 21(4):10056610.1016/j.repbio.2021.10056634626941 [Google Scholar] [CrossRef] [PubMed]
[85]. Sun J, Zhang D, Xu J, Chen C, Deng D, Pan F, Circulating FABP4, nesfatin-1, and osteocalcin concentrations in women with gestational diabetes mellitus: A meta-analysisLipids Health Dis 2020 19(1):19910.1186/s12944-020-01365-w32861247 [Google Scholar] [CrossRef] [PubMed]
[86]. Martinez-Portilla RJ, Villafan-Bernal JR, Lip-Sosa DL, Meler E, Clotet J, Serna-Vela FJ, Osteocalcin serum levels in gestational diabetes mellitus and their intrinsic and extrinsic determinants: Systematic review and meta-analysisJ Diabetes Res 2018 2018:498673510.1155/2018/498673530693288 [Google Scholar] [CrossRef] [PubMed]
[87]. Bawah AT, Seini MM, Abaka-Yawason A, Alidu H, Nanga S, Leptin, resistin and visfatin as useful predictors of gestational diabetes mellitusLipids Health Dis 2019 18(1):22110.1186/s12944-019-1169-231836012 [Google Scholar] [CrossRef] [PubMed]
[88]. Hu SM, Chen MS, Tan HZ, Maternal serum level of resistin is associated with risk for gestational diabetes mellitus: A meta-analysisWorld J Clin Cases 2019 7(5):585-99.10.12998/wjcc.v7.i5.58530863758 [Google Scholar] [CrossRef] [PubMed]
[89]. Radzicka S, Pietryga M, Iciek R, Brązert J, The role of visfatin in pathogenesis of gestational diabetes (GDM)Ginekol Pol 2018 89(9):518-21.10.5603/GP.a2018.008830318580 [Google Scholar] [CrossRef] [PubMed]
[90]. Ruidar SB, Mushtaq M, Raza SA, Mukhtar F, Rehman T, Ning W, Visfatin as a Biomarker for Early Detection of Gestational Diabetes MellitusPak J Med Health Sci 2022 16(7):1000-02.10.53350/pjmhs221671000 [Google Scholar] [CrossRef]
[91]. Vorobjova T, Tagoma A, Talja I, Janson H, Kirss A, Uibo R, FABP4 and I-FABP levels in pregnant women are associated with body mass index but not gestational diabetesJ Diabetes Res 2022 2022:108943410.1155/2022/108943435647197 [Google Scholar] [CrossRef] [PubMed]
[92]. Ron I, Mdah R, Zemet R, Ulman RY, Rathaus M, Brandt B, Adipose tissue-derived FABP4 mediates glucagon-stimulated hepatic glucose production in gestational diabetesDiabetes Obes Metab 2023 25(11):3192-201.10.1111/dom.1521437449442 [Google Scholar] [CrossRef] [PubMed]