Dental implants have established themselves as a conventional therapy for replacing missing teeth and facilitating oral restoration in patients with partial or complete edentulism. Although implant-supported restorations have high success rates, they are still prone to complications, failures, and certain limitations [1].
Common complications in implant dentistry include peri-implant mucositis, peri-implantitis, and screw loosening, which typically result in inflammation of the peri-implant tissues and, ultimately, the loss of supporting crestal bone. Bacterial microleakage at the IAI has been identified as one of the primary causes of peri-implantitis. The IAI may significantly contribute to crestal bone loss due to the micro gap between the implant and the abutment in two-piece implant systems [2].
Intraoral bacteria and fluid can ingress into the inner implant space through the IAI micro gap, creating a favourable environment for toxins. The discharge of these substances back through the IAI and the subsequent activation of the adjacent bone tissue may result in alveolar bone loss and peri-implantitis, ultimately culminating in implant failure [3].
The screw is crucial to the implant/abutment assembly, playing a key role in the mechanical performance of screw-retained restorations. The attachment of the implant/abutment assembly relies on the mechanical force generated by the retaining screw, which depends on the rotational force (i.e., torque) applied to it [4]. Screw-retained implant-supported prostheses often face biomechanical issues, including screw loosening, which is particularly prevalent in single restorations [1]. This leads to instability of the prosthetic component [4].
To reduce IAI bacterial microleakage and screw loosening, sealing gel has been used to close the IAI micro gap, along with anti-loosening agents [3,5,6]. Sealing gel consists of a silicone matrix, a highly viscous, hydrophobic material that maintains its consistency and provides a hermetic seal without hardening [3]. While previous studies have focused on the influence of mechanical factors, such as the implant-abutment fit, on microleakage, research on chemical or gel-based sealing methods has been relatively limited [1,7,8]. Although in-vitro studies have examined the microleakage and mechanical properties of sealing gel, in-vivo effects have yet to be fully explored [2,3,7]. Consequently, the present study investigated the bacterial microleakage of sealing gel in the oral environment. The aim of the current study was to compare the bacterial microleakage at the implant-abutment junction with and without sealing gel (conventional method).
Materials and Methods
The Split-mouth randomised controlled trial was conducted at the Department of Prosthodontics and the Department of Microbiology at Rural Dental College and Rural Medical College, Pravara Institute of Medical Sciences (Deemed to be University), Ahmednagar, Maharashtra, India, from April 2023 to October 2023. The study protocol received approval from the Institutional Ethical Committee at Pravara Institute of Medical Sciences, with the ethical clearance number DR/IEC, PIMS-DU/2023/241. The study was registered in the Clinical Trial Registry India (CTRI) with the number CTRI/2023/08/056992.
Patients were informed about the nature of the study, and willing participants were asked to sign the informed consent form before the study commenced.
Inclusion criteria: Patients requiring or indicating the need for implant-supported prostheses, as well as those who had previously received two or more dental implants, were included in the study. Patients who had undergone first-stage implant placement were also included, as the sealing gel was applied during the second stage of the procedure. In patients with two or more implants, one implant was treated with the sealing gel, while the other served as a control without the gel, allowing for a comparison within the same patient. The study utilised standardised Dentium Superline R dental implants, which feature a hexagonal internal channel.
Exclusion criteria: Patients with poor oral hygiene and those with parafunctional habits were excluded from the study.
Sample size calculation: A sample size of 30 was calculated using the formula:
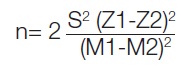
where,
M1 (Mean test intervention): 17.71
This indicates the average value or outcome for the group of implants where the sealing gel (test intervention) was applied.
M2 (Mean control intervention): 24.00
This represents the average value for the group of implants where no sealing gel (control intervention) was applied.
S1 (Standard deviation of M1): 3.73
S2 (Standard deviation of M2): 3.26
Z1 (associated with alpha, typically 1.64485 for a one-sided test)
Z2 (associated with beta, typically 0.84162 for a power of 0.8)
The minimum sample size was 30, with 15 participants in each group [3]. This was calculated using the AP Kulkarni software.
Study Procedure
A total of 30 implant samples were included in the study based on the established inclusion and exclusion criteria, with participants’ ages ranging from 20 to 50 years. The sample consisted of nine males and six females. The samples were divided into two groups (A and B), with 15 implant samples in each group. In Group A, Technovent medical-grade silicone was used as a sealing gel between the implants, while in Group B, no sealing gel was placed between the implants [Table/Fig-1].
Consodilated Standards of Reporting Trails (CONSORT) flow diagram.
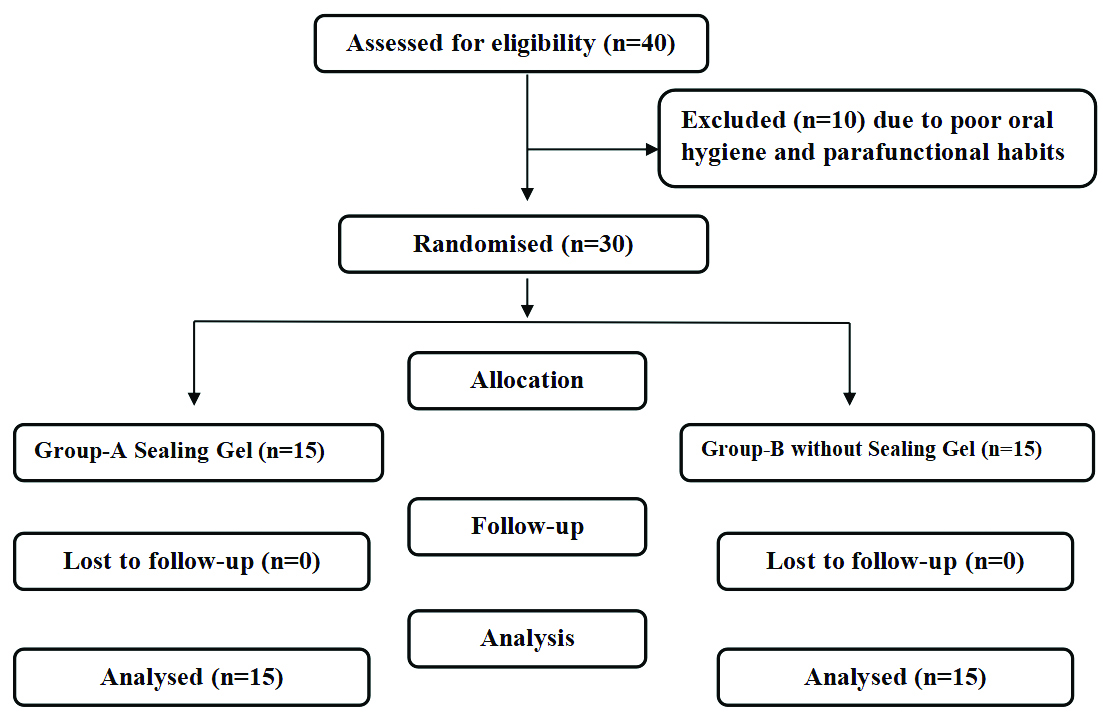
For the randomisation of patients undergoing implant treatment, each eligible patient who opted for the treatment participated in a random lottery method. Patients were asked to draw a lottery chit from four options: RS (Right-side with sealing gel), RW (Right-side without sealing gel), LS (Left-side with sealing gel), and LW (Left-side without sealing gel). This approach ensured unbiased assignment of patients to different treatment groups, facilitating a fair comparison of outcomes across the RS, RW, LS, and LW options.
Outcome measures: Bacterial microleakage, with and without sealing gel, was assessed by counting CFUs. This assessment was conducted 15 to 20 days after the placement of the sealing gel in Group A, while Group B did not receive any sealing gel.
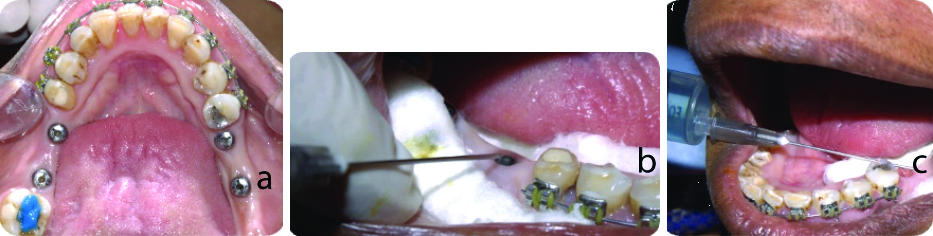
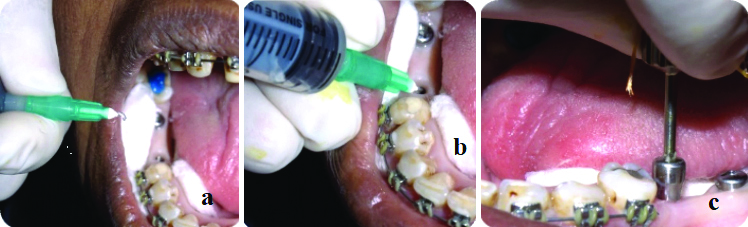
Second stage surgery/implant level impression and sterile field assessment: The implant site was isolated using sterile cotton rolls and suction. During the second stage of surgery, when taking the implant-level impression, the internal channel of the implant was sterilised with a chlorhexidine solution for 30 seconds to two minutes in both groups A and B [Table/Fig-2a-c].
a) Sample site; b, c) Sterilisation of internal compartment with chlorhexidine solution for both groups A and B.
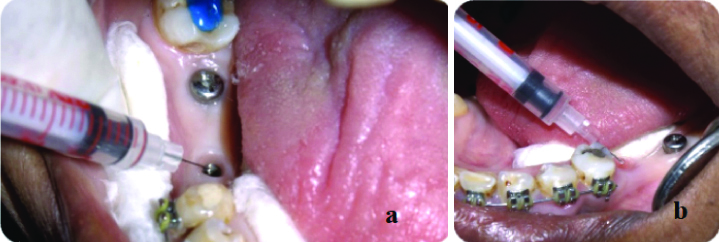
The sterile environment was assessed by introducing 10 μL of sterile saline into the internal cavity of the implant using an insulin syringe. It was determined prior to the initiation of the study that the internal volume of the implants could accommodate a saline volume of 10 μL. The saline was then immediately drawn back into the syringe for microbial assessment by culturing the saline sample to evaluate the sterile field. A new sterile insulin syringe was used for each sample [Table/Fig-3a,b] [9].
a,b) Injecting 10 μL of sterile saline into the internal compartment of the implant with Insulin syringe and immediately drawn back into syringe for microbial assessment for both groups A and B.
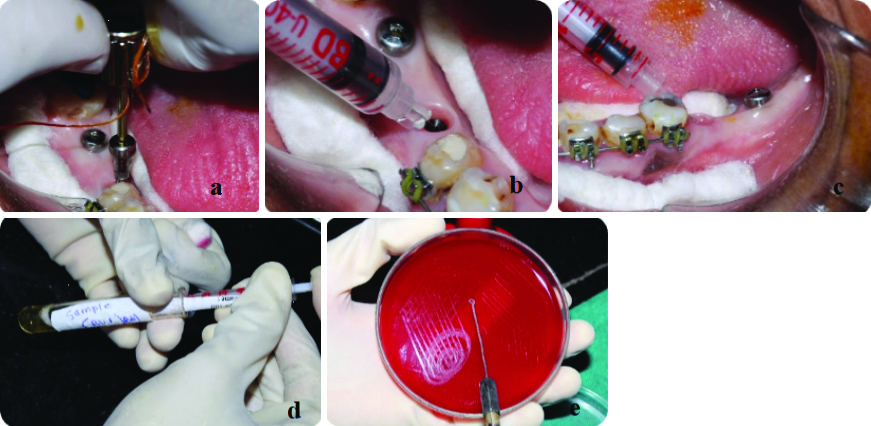
After the saline sample was drawn back from both groups, sealing gel (Technovent medical-grade silicone) was injected into the internal compartment of Group A, followed by the placement of a healing abutment. In Group B, a healing abutment was placed without injecting sealing gel (conventional method) [Table/Fig-4a-c].
a,b) Injecting sealing gel in Group A; c) Healing abutment was placed without injecting sealing gel.
Saline samples from groups A and B were cultured on Blood Agar (BA) and incubated at 37°C under aerobic conditions for 24 hours to assess sterility. Samples that showed no growth on the BA were included for further bacterial microleakage assessment after the sealing gel was injected, while samples that exhibited growth were excluded from the bacterial microleakage assessment due to contamination from improper sterilisation [Table/Fig-5].
Microbiological analysis-Sterile field assessment (both A and B groups).

Bacterial microleakage assessment: The healing abutment was removed after full soft tissue healing, which occurred within 15 to 20 days. Subsequently, a sterile insulin syringe was used to introduce sterile saline into the internal compartment of the implant. The saline was promptly withdrawn and transferred into a sterile Brain Heart Infusion (BHI) Broth, which served as an enriched medium for microorganism culturing. Following a 24-hour incubation period, samples were cultured on BA plates and incubated at 37°C under aerobic conditions for an additional 24 hours [Table/Fig-6a-e].
a-c) Bacterial Microleakage assessment (after 15 -20 days); d) Saline sample were incubated in Brain Heart Infusion (BHI) Broth for 24 hr; e) After an incubation period of 24 hours, samples were cultured on Blood Agar (BA) plates.
This procedure was undertaken to evaluate bacterial microleakage at the IAI in both groups A and B. The microbial assessment was meticulously performed, determining the type of colony and the colony count using Colony Forming Units (CFUs).
Statistical Analysis
Data entry was performed using Microsoft Excel spreadsheets, and descriptive and inferential statistical analyses were conducted using SYSTAT version 12 (developed by Crane’s Software, Bengaluru, a licensed copy). Statistical analysis was carried out using descriptive statistics as percentage proportions. All assessment variables under study were compared using Chi-square and Mann-Whitney’s U tests.
Results
The randomised controlled trial included 30 implant patients with a mean age of 35±1 years, ranging from 20 to 50 years. The gender distribution was nine males and six females. A bacterial microleakage assessment of 30 samples was performed for Group A (n=15) (with sealing gel) and Group B (n=15) (without sealing gel) after 15 days of healing abutment placement. The type and number of colonies for both groups were assessed using the CFUs method, and the values were recorded, tabulated, and compared. In Group A, none of the samples showed growth of any bacterial colonies; however, in Group B, 11 samples exhibited growth of bacterial colonies [Table/Fig-7].
Bacterial microleakage at healing abutment junction (AS- Group A Sealing gel, BU-Group B Unsealed-without sealing gel).
| Group A Sealing gel | Group B Conventional method |
|---|
| Sr. No | Type/No. of colony | Sr. no | Type of colony | No. of colony |
|---|
| Sample 1 AS | Sterile | Sample 1BU | Enterococcus | 500> |
| Sample 2 AS | Sterile | Sample 2BU | Enterococcus | 500 |
| Sample 3 AS | Sterile | Sample 3BU | Growth of bacilli-Gram+ | 1000> |
| Sample 4 AS | Sterile | Sample 4BU | Enterococcus | 500> |
| Sample 5 AS | Sterile | Sample 5BU | Enterococcus | 250 |
| Sample 6 AS | Sterile | Sample 6BU | Enterococcus | 500 |
| Sample 7 AS | Sterile | Sample 7BU | Sterile | - |
| Sample 8 AS | Sterile | Sample 8BU | Growth of bacilli-Gram+ | 1000> |
| Sample 9 AS | Sterile | Sample9 BU | Growth of bacilli-Gram+ | 500 |
| Sample 10 AS | Sterile | Sample10 BU | Sterile | - |
| Sample 11 AS | Sterile | Sample11 BU | Growth of bacilli-Gram+ | 500> |
| Sample 12 AS | Sterile | Sample 12 BU | Enterococcus | 250 |
| Sample 13 AS | Sterile | Sample 13 BU | Sterile | |
| Sample 14 AS | Sterile | Sample 14 BU | Sterile | |
| Sample 15 AS | Sterile | Sample 15 BU | Enterococcus | 250 |
| Total (sterile samples- AS) | 15 | Total (sterile samples- BU) | 4 | 4 |
| Total (colonies detected- AS) | NIL | Total (colonies detected- BU) | 11 | 11 |
Two groups were compared regarding bacterial microleakage at the implant healing abutment junction. Group A, which utilised a sealing gel, demonstrated 100% sterility, indicating the absence of any colony formation. In contrast, Group B, which followed the conventional method without a sealing gel, exhibited only 4 (26.67%) samples that were sterile. Additionally, 7 (46.67%) samples showed growth of enterococcus, and 4 (26.67%) samples showed growth of gram-positive bacilli. The Mann-Whitney’s U test indicated a significant difference (p=0.001) between the two groups, highlighting the superior efficacy of the sealing gel in reducing bacterial microleakage compared to the conventional method that does not use a sealing gel [Table/Fig-8].
Comparison of type of colony in Group A and Group B.
| Type of colony | Group A Sealed (n=15) | Group B Conventional method (n=15) | p-value |
|---|
| n (%) | n (%) |
|---|
| Sterile | 15 (100%) | 4 (26.67%) | 0.001* |
| Enterococcus | 0 | 7 (46.66%) |
| Growth of bacilli(Gram-postive) | 0 | 4 (26.67%) |
| Total | 15 (100%) | 15 (100%) | |
| p-value | - | 0.549 | |
*p-value analysed using Mann-Whitney’s U test
A significant variation in the number of colonies was detected between the groups (p=0.001). However, the intragroup analysis in Group B indicated that the difference in the proportion of the number of colonies was not statistically significant (p=0.955) [Table/Fig-9].
Comparison of number of colonies in Group A and Group B.
| Number ofcolonies | Group Asealed (n=15) | Group Bconventional method (n=15) | p-value |
|---|
| n (%) | n (%) |
|---|
| Nil | 15 (100%) | 4 (26.67%) | 0.001* |
| 250 | 0 | 3 (20%) |
| 500 | 0 | 3 (20%) |
| 500> | 0 | 3 (20%) |
| 1000> | 0 | 2 (13.33%) |
| Total | 15 (100%) | 15 (100%) | |
| p-value | - | 0.955 | |
*p-value anlaysed using Mann-Whitney’s U test
Discussion
The use of dental implants to restore partially and fully edentulous arches has become a common and vital approach for enhancing patients’ overall well-being. The effectiveness of these implant restorations depends significantly on both biological and mechanical factors [10-12]. Oral implants are usually implemented in a two-stage process: first, the fixture is surgically inserted, and then, after osseointegration, the transmucosal abutment is attached to the fixture [10,13,14].
The internal hexagonal connection, where a segment of the abutment fits into the implant body, is currently the most widely used configuration in two-piece implant systems [15]. Forces applied to the prosthetic components can impact the micro-movements or bending of IACs, leading to an increase in the microgap and causing a “pump effect” between the implant and the surrounding peri-implant tissues. Microbial colonisation usually impacts the peri-implant sulci, the external surfaces of implants, and the internal cavities of two-phase dental implants, all of which are vulnerable to bacterial contamination [16]. The subgingival gap within the implant components serves as an optimal site for plaque retention. This gap ranges from 1 to 49 mm, providing ample space for microbial leakage [17].
Complications associated with the microgap at the IAI can be either biological issues such as peri-implantitis, peri-implant mucositis, crestal bone resorption, and halitosis; or mechanical problems like abutment/implant fracture or abutment screw loosening. Bacteria can invade and establish themselves in the gaps within the IAI, releasing harmful substances and byproducts into the surrounding tissues. Microleakage may allow the entry of fluids, microorganisms, molecules, and ions into the IAI, potentially leading to biological and mechanical complications, including screw loosening [18].
Literature has confirmed that microbial flora within the implant cavity can arise from contamination during implant placement or from microorganisms introduced from the oral environment after prosthesis insertion. Anti-infective treatment approaches are beneficial for preventing peri-implantitis [17]. Suggested treatment methods for addressing contaminated internal implant interfaces include mechanical, chemical, and physical techniques. However, the current scientific literature does not provide enough evidence to support a specific treatment protocol [17].
Various methods have been proposed to reduce or prevent bacterial contamination at the IAI. These include using sealant materials, cleaning the internal cavity of the implant, and employing shape memory alloys. The idea of platform switching, introduced by Lazzara RJ and Porter SS, suggests that a slimmer abutment can increase the distance between the implant-abutment junction and the crestal bone. This adjustment aims to establish an appropriate biological width that minimises microbial contamination and bone loss [19]. A range of materials has been suggested for sealing IAIs, such as adhesives, silicone O-rings, silicone sealing washers, chlorhexidine-thymol varnish, and a 2% chlorhexidine solution [20].
Silicone matrix is a highly viscous material that forms a hermetic seal and exhibits hydrophobic characteristics, allowing it to maintain its consistency without hardening. This property is crucial as it prevents microleakage and bacterial colonisation around the implant site, reducing the risk of peri-implant diseases and minimising the potential for complications such as loss of crestal bone. The use of a silicone matrix contributes to additional screw stability, mitigating occlusal stress and preventing abutment screw loosening [1]. Its hydrophobic nature also discourages the development of putrid odours, ensuring a more hygienic and comfortable environment for the patient [21].
The present study found a significant difference in bacterial microleakage between implants with and without sealing gel. Group A (with sealing gel) was 100% sterile, while Group B (without sealing gel) showed 26.67% sterility, 46.66% growth of enterococcus, and 26.67% growth of gram-positive bacilli (p=0.001), emphasising the effectiveness of the sealing gel in preventing bacterial microleakage compared to the conventional method without sealing gel.
The effect of the sealing gel varied between the two groups, decreasing IAI microleakage only for implants in which sealing gel was injected. This indicates that the sealing gel is particularly beneficial for designs with poorer sealing properties. The application of sealing gel plays a crucial role in minimising the occurrence of gaps and microleakage at the IAI. The viscous nature of the sealing material facilitates a tight and hermetic seal. A greater volume of sealing gel in the implant’s IAI microgap might explain the reduction in microleakage [3,21]. Yu P et al., and Smojver I et al., suggested that using sealing gel could enhance the longevity of the implant. In contrast, without sealing materials, the potential for leakage significantly increases due to incomplete adaptation and microgaps in the implant-abutment components [3,21].
A study conducted by Nayak AG et al., and Zarbaksh A et al., proposed the replacement of GapSeal every five years. This recommendation stems from the observation that as GapSeal degrades, its ions are released into the peri-implant tissues. Consequently, assessing the longevity of the material’s effectiveness becomes crucial. Moreover, the influence of oral fluids on the outcomes warrants examination. Parameters such as microbial leakage and fatigue testing might exert diverse effects on the interface [2,7].
Yu P et al., highlighted that the utilisation of silicone gel could enhance both the immediate securing and long-term resistance to loosening of three implant screw thread connections. This intervention also led to a decrease in IAI microleakage within the Straumann system and a reduction in abutment screw thread wear in the Nobel and Wego systems. No exacerbation in IAI microleakage or thread abrasion was observed in other implant systems. The tested silicone sealing gel demonstrates potential in mitigating the risk of biomechanical complications associated with implant restorations and may prolong their lifespan [3].
The internal surface of the implant can serve as a habitat for bacterial colonisation, potentially causing tissue damage and peri-implant tissue infections. To mitigate the ingress of microorganisms into these regions, Duarte AR et al., advocated for the application of silicone sealant and chlorhexidine varnish in the cervical areas of dental implants. This method remained effective for more than 35 days and demonstrated prolonged prevention of microleakage. Therefore, silicone sealant and chlorhexidine varnish could complement the placement of sealing gel at the IAI [20].
Groenendijk E et al., found that using a 0.2% Chlorhexidine (CHX) solution during second-stage surgery suppressed bacterial growth on the fixtures, with this positive effect lasting upto six weeks. Additionally, CHX gel demonstrated a more prolonged antimicrobial effect in the subgingival environment than CHX solution [22].
The study demonstrated that sealing gel significantly reduced microleakage at the IAI, especially in designs with inherently poorer sealing properties. The application of sealing gel could enhance the longevity of implants by improving their sealing effectiveness. Conducting similar clinical studies to evaluate the duration of the seal provided by sealing gel and its combination with an antimicrobial agent can yield valuable insights into implant maintenance and longevity.
Limitation(s)
The use of final prosthesis abutments would allow for a more comprehensive analysis of the sealing effect, which was not conducted in present study. Additionally, incorporating an antimicrobial agent into the sealing gel could provide added benefits.
Conclusion(s)
Sealing gel effectively reduces bacterial microleakage at the IAI. It decreases microleakage and can contribute to the longevity of implants, thereby benefiting patients’ long-term oral health outcomes. The application of sealing gel plays a crucial role in maintaining a tight seal, preventing microbial colonisation, and minimising the risk of peri-implant diseases.
*p-value analysed using Mann-Whitney’s U test
*p-value anlaysed using Mann-Whitney’s U test