A Case of Celiac Disease with Pericardial Effusion
Yogesh Kautikrao Kakde1, Shilpa Abhay Gaidhane2, Sourya Acharya3, Sunil Kumar4, Shubham Nimkar5
1 Postgraduate Resident, Department of General Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research (Deemed to be University), Wardha, Maharashtra, India.
2 Professor, Department of General Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research (Deemed to be University), Wardha, Maharashtra, India.
3 Professor, Department of General Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research (Deemed to be University), Wardha, Maharashtra, India.
4 Professor, Department of General Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research (Deemed to be University), Wardha, Maharashtra, India.
5 Postgraduate Resident, Department of General Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research (Deemed to be University), Wardha, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Etymology: Author Origin Dr. Yogesh Kautikrao Kakde, Postgraduate Resident, Department of General Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research (Deemed to be University), Wardha-442107, Maharashtra, India.
E-mail: yogeshkakde95@gmail.com
Celiac disease is a unique enteropathic immune disorder and is now considered a distinct disease entity with protein manifestation and worldwide distribution. The deposition of immune complexes in the small intestine could be a possible reason for the extraintestinal autoimmune manifestations of Celiac disease. Pericardial effusion, though rare in adults, is likely a result of these autoimmune disorders related to Celiac disease. In present study case report, the authors present the case of a 67-year-old female patient with generalised oedema, anasarca, and pitting oedema in the lower extremities. Due to multiple episodes of vomiting, upper gastrointestinal endoscopy was performed, which revealed atrophic duodenal mucosa, and duodenal biopsies were taken from the second part of the duodenum (D2 biopsies). The possibility of Celiac disease was considered, and anti-deamidated gliadin peptide, Immunoglobulin A (IgA), and Immunoglobulin G (IgG) antibodies were sent. Celiac disease was diagnosed based on elevated serum levels of anti-deamidated gliadin peptide, IgA, and IgG antibodies, as well as histologic findings in the small bowel (duodenal biopsies D2). During the thorough evaluation, pericardial effusion was incidentally detected. Celiac disease is an autoimmune disorder in which exposure to gluten triggers inflammation in genetically predisposed individuals. The illness is estimated to have a prevalence of 2%, and most affected people remain undiagnosed. Pericardial effusion is a possible manifestation of Celiac disease, but there is currently no evidence that the disease directly causes the effusion.
Anaemia, Immune disorder, Malabsorption, Oedema
Case Report
A 67-year-old female patient presented with a chief complaint of easy fatigability, swelling in both lower limbs, weight loss, distension of the abdomen, abdominal pain, vomiting, and loss of appetite for three months and was admitted to the emergency room. The patient had a history of multiple hospital admissions for the same symptoms. There was no history of fever, haematuria, jaundice, or chest pain. On examination, the patient was conscious. She was afebrile with a pulse of 110 beats per minute and a blood pressure level of 100/60 mmHg. The patient’s jugular venous pressure was not raised. Bilateral pitting pedal oedema, pallor, and tachypnea were observed, but there was no icterus, cyanosis, or clubbing. On physical examination, the conjunctiva was pale. On cardiovascular examination, the heart sounds were muffled. The respiratory system examination was normal, and abdominal examinations were normal. Pitting oedema was seen in bilateral lower limbs. Chest and abdominal examinations were normal. A 2D echocardiogram (echo) was suggestive of moderate pericardial effusion of approximately 18 mm, mildly dilated left atria and left ventricle, and no regional wall motion abnormality with an ejection fraction of 45%. Diagnostic pericardiocentesis was performed, and pericardial fluid was sent for microbiological culture examination, which indicated the presence of 1-2/high power field (hpf) pus cells on Gram staining, but no organism was identified. Arterial blood gas analysis was done, and the results were within normal limits. Other blood analysis results are shown in [Table/Fig-1].
| Parameters | Results | Reference values |
|---|
| Haemoglobin (g/dL) | 7.2 | 11-14 |
| TLC counts (cell/mm3) | 5800 | 4000-11000 |
| Haematocrit (%) | 28 | 30-34 |
| MCV (fL) | 70 | 85-110 |
| Inflammatory parameters |
| Anti-deamidatedgliadin peptide-IgA (AU/mL) | 38.54 | Negative: <15Equivocal: 15-30Positive: >30 |
| Anti-deamidatedgliadin peptide-IgG (AU/mL) | 31.1 | 0.9 (deficitive)>1.1 (positive)0.9-1.1 (borderline) |
| Erythrocyte sedimentation rate (mm/hr) | 12 | 0-15 |
| C-reactive protein (mg/dL) | 3 | <1 |
| Kidney function test and serum electrolytes |
| Potassium (mmol/L) | 3.1 | 3.5-5.5 |
| Calcium (mg/dL) | 7.1 | 8.4-10.2 |
| Magnesium (mg/dL) | 1.1 | 1.6-2.2 |
| Sodium (mmol/L) | 114 | 137-145 |
| Creatinine (mg/dL) | 0.5 | 0.5-1.04 |
| Urea (mg/dL) | 14 | 7-17 |
| Liver function test |
| Alkaline phosphatase (U/L) | 42 | 38-126 |
| ALT (SGPT) (U/L) | 28 | <35 |
| AST (SGOT) (U/L) | 30 | 14-36 |
| Total protein (g/dL) | 6.3 | 6.2-8.2 |
| Albumin (g/dL) | 3 | 3.5-5 |
| Total bilirubin (mg/dL) | 0.9 | 0.2-1.3 |
| Iron parameters |
| Ferritin (ng/mL) | 92 | 6.4-136 |
| Total iron binding capacity (mcg/dL) | 262 | 240-450 |
| Vitamin B12 (ng/mL) | 320 | 239-931 ng/mL |
| Thyroid profile |
| TSH (μIU/mL) | 1.6 μIU/ML | 0.4-4.68 |
| FT3 (pg/dL) | 4.2 pg/mL | 2.7-5.2 |
| FT4 (ng/dL) | 1.54 ng/dL | 0.7-2.19 |
| Urine analysis |
| Urine albumin | Nil |
| Urine sugar | Nil |
| Pus cells | Nil |
| Epithelial cells | Nil |
| RBC | Nil |
| NT-proBNP | 302 pg/mL (Normal range: Lower than 450 pg/mL) |
TLC: Total leucocyte count; MCV: Mean corpuscular volume; ALT: Alanine transaminase; SGPT: Serum glutamic pyruvic transaminase; SGOT: Serum glutamic oxaloacetic transaminase; FT3: Free triiodothyronine; FT4: Free thyroxine; RBC: Red blood cells; NT-proBNP: N-terminal pro-B-type natriuretic peptide
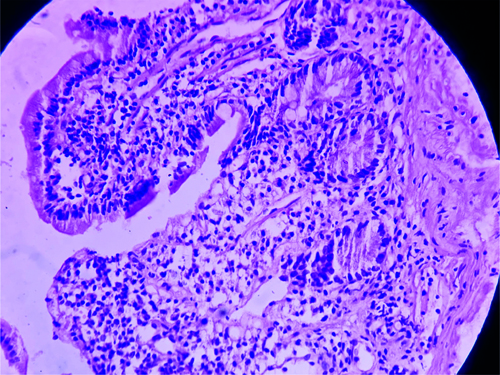
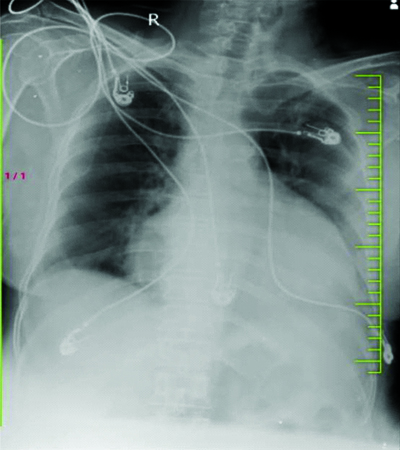
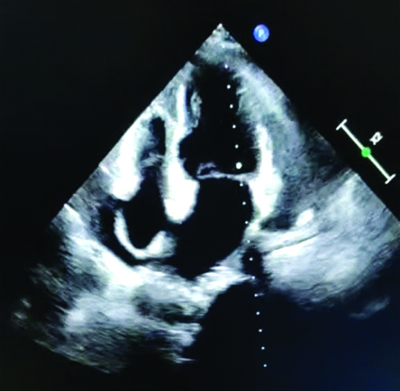
After history taking, clinical examinations, and routine blood investigations, as well as additional investigations like 2D echo and chest X-ray, it was concluded that kidney disease was ruled out as the kidney function tests were normal. The chest X-ray showed cardiomegaly, leading to the decision to perform a 2D echo, which revealed moderate pericardial effusion of approximately 18 mm, mildly dilated left atria and left ventricle, and no regional wall motion abnormality with an ejection fraction of 45%. Hypothyroidism was also ruled out as the cause of the pericardial effusion, despite normal Thyroid Stimulating Hormone (TSH) levels. Celiac disease was considered as a possible cause, given the patient’s anaemia and hypoalbuminemia (haemoglobi level of 7.2 g/dL and serum albumin level of 3 g/dL on admission). The patient received transfusions of three packed red blood cells on three consecutive days and 20% intravenous albumin to address the anasarca. This resolved the causes of anasarca and corrected the hyponatremia (mmol/L) that was likely due to dilutional hyponatremia caused by the anasarca. On follow-up after one a month, the patient was doing well, with improved appetite and diminished oedema. Follow-up investigations, such as haemoglobi, serum albumin, serum sodium, and 2D echo, were normal. Upper gastroesophageal endoscopy revealed atrophic duodenal mucosa. Multiple biopsies were obtained, which on histolgical section (Haematoxylin and Eosin stain {H&E}). showed atrophy of the mucosal layer of the duodenum with focal loss of villi and dense infiltration of lymphocytes forming lymphoid follicles, along with a few plasma cells and immunoblasts, supporting the diagnosis of Celiac disease (as shown in [Table/Fig-2]). The routine chest X-ray also indicated globular enlargement of the heart, suggestive of pericardial effusion. This was confirmed by the 2D echo, which showed moderate pericardial effusion (as shown in [Table/Fig-3] and [Table/Fig-4].
Shows atrophy of the mucosal layer of the duodenum with focal loss of villi, dense infiltration of lymphocytes forming lymphoid follicles, few plasma cells, and few [H&E 40X].
Globular enlargement of heart, suggesting of pericardial effusion.
A 2D echo suggestive of moderate pericardial effusion approximately 18 mm, 140 mildly dilated left atrial, left ventricular, no Regional Wall Motion Abnormalities Ejection Fraction (RWMA EF).
Discussion
Celiac disease is associated with several disorders, such as cardiovascular disease. There is a direct link between Celiac disease and a high incidence of dilated cardiomyopathy, which can eventually lead to pericardial effusion. Celiac disease is an autoimmune disorder triggered by exposure to gluten in genetically predisposed individuals. It is estimated to have a prevalence of 2%, and most affected individuals remain undiagnosed [1]. While pericardial effusion is a possible manifestation of Celiac disease, there is currently no evidence that the disease directly causes the effusion. Many individuals with Celiac disease go undiagnosed. In children, characteristic signs of the disease include malabsorption, steatorrhea, weight loss, and failure to thrive [2]. However, adults can develop the condition at any age without these traditional childhood symptoms. Adults are more likely to present with anaemia as the first sign of illness [3], primarily due to iron malabsorption, although nutritional deficiencies and chronic illnesses can also contribute to anaemia.
The occurrence of pericardial effusion may be explained by molecular mimicry, where T lymphocytes are triggered by gliadin or tissue transglutaminase and cross-react with self-antigens. Transglutaminase forms neoantigens by cross-linking or deamidating external or self-antigens [4]. Advancements in diagnostic facilities have led to an increased diagnosis of latent and atypical Celiac disease. This case adds to our understanding of the various presentations of Celiac disease. Based on histologic and serologic results, Celiac disease was diagnosed in our patient. Although the patient was asymptomatic, an incidental finding of pericardial effusion measuring 18 mm was detected on 2D echo. Pericardiocentesis was performed based on the cardiologist’s recommendation due to the large volume of pericardial effusion. The two evident abnormalities in the patient were anaemia and anasarca. Notably, Celiac disease is a prevalent cause of iron deficiency anaemia [5,6]. There is a wide range of ailments associated with Celiac disease, including cardiovascular disease. Increased risks of autoimmune myocarditis, dilated cardiomyopathy [7], atrial fibrillation, cardiovascular mortality [8], and ischaemic heart disease have all been linked to Celiac disease [9].
Research has shown that 50% of children with Celiac disease have a small, asymptomatic pericardial effusion that can only be detected using specific instruments. The only effective treatment for Celiac disease is a gluten-free diet and nutritional therapy, including dietary supplements such as calcium and vitamins. After therapeutic pericardiocentesis, the patient’s clinical condition improved [10].
Conclusion(s)
Celiac disease may have mild effects on health; however, in some cases, it can be complicated by rare but life-threatening events such as pericardial effusion. Failing to respond rapidly to these bodily changes can lead to fatalities.
TLC: Total leucocyte count; MCV: Mean corpuscular volume; ALT: Alanine transaminase; SGPT: Serum glutamic pyruvic transaminase; SGOT: Serum glutamic oxaloacetic transaminase; FT3: Free triiodothyronine; FT4: Free thyroxine; RBC: Red blood cells; NT-proBNP: N-terminal pro-B-type natriuretic peptide
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 14, 2023
Manual Googling: Jul 11, 2023
iThenticate Software: Sep 21, 2023 (7%)
[1]. Dubé C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C, The prevalence of Celiac disease in average-risk and at-risk Western European populations: A systematic reviewGastroenterology 2005 128(4):S57-67.10.1053/j.gastro.2005.02.01415825128 [Google Scholar] [CrossRef] [PubMed]
[2]. Andersen DH, Celiac syndrome: Vi. The relationship of Celiac disease, starch intolerance, andsteatorrheaThe Journal of Paediatrics 1947 30(5):564-82.10.1016/S0022-3476(47)80050-220297412 [Google Scholar] [CrossRef] [PubMed]
[3]. Rodrigo-Sáez L, Fuentes-Álvarez D, Pérez-Martínez I, Álvarez-Mieres N, Niño-García P, de-Francisco-García R, Refractory iron-deficiency anemia and gluten intolerance-response to gluten-free dietRevista Espanola de Enfermedades Digestivas 2011 103(7):349-54.10.4321/S1130-0108201100070000321770680 [Google Scholar] [CrossRef] [PubMed]
[4]. Fasano A, Clinical presentation of Celiac disease in the paediatric populationGastroenterology 2005 128(4):S68-73.10.1053/j.gastro.2005.02.01515825129 [Google Scholar] [CrossRef] [PubMed]
[5]. Ackerman Z, Eliakim R, Stalnikowicz R, Rachmilewitz D, Role of small bowel biopsy in the endoscopic evaluation of adults with iron deficiency anaemiaAm J Gastroenterol 1996 91(10):2099-102. [Google Scholar]
[6]. Unsworth DJ, Lock FJ, Harvey RF, Iron-deficiency anaemia in premenopausal womenThe Lancet 1999 353(9158):110010.1016/S0140-6736(05)76459-X10199377 [Google Scholar] [CrossRef] [PubMed]
[7]. Emilsson L, Andersson B, Elfström P, Green PH, Ludvigsson JF, Risk of idiopathic dilated cardiomyopathy in 29 000 patients with Celiac diseaseJournal of the American Heart Association 2012 1(3):e00159410.1161/JAHA.112.00159423130142 [Google Scholar] [CrossRef] [PubMed]
[8]. Emilsson L, Smith JG, West J, Melander O, Ludvigsson JF, Increased risk of atrial fibrillation in patients with Celiac disease: A nationwide cohort studyEuropean Heart Journal 2011 32(19):2430-37.10.1093/eurheartj/ehr16721653560 [Google Scholar] [CrossRef] [PubMed]
[9]. Frustaci A, Cuoco L, Chimenti C, Pieroni M, Fioravanti G, Gentiloni N, Celiac disease associated with autoimmune myocarditisCirculation 2002 105(22):2611-18.10.1161/01.CIR.0000017880.86166.8712045166 [Google Scholar] [CrossRef] [PubMed]
[10]. Hopman EG, le Cessie S, von Blomberg BM, Mearin ML, Nutritional management of the gluten-free diet in young people with Celiac disease in The NetherlandsJournal of Paediatric Gastroenterology and Nutrition 2006 43(1):102-08.10.1097/01.mpg.0000228102.89454.eb16819385 [Google Scholar] [CrossRef] [PubMed]