In recent decades, the global healthcare landscape has witnessed a concerning rise in fungal infections, especially among patients receiving medical care in hospitals [1]. These infections not only prolong hospital stays but also create significant financial burdens [2]. Candida, a genus of yeast, is a key contributor to approximately 70% of fungal infections within hospital settings [3]. Candidiasis, the disease caused by Candida species, has emerged as a major public health concern due to its high rates of morbidity and mortality [4]. Traditionally, Candida albicans was the primary culprit, responsible for 60-80% of fungal infections. However, Non albicans Candida (NAC) species are now taking the lead as predominant pathogens. This shift is partly attributed to the widespread use of antibiotics and steroids, which have contributed to the global surge in fungal infections [5].
Simultaneously, TB remains a significant global public health challenge, afflicting approximately 10.6 million individuals annually and resulting in 1.6 million deaths [6]. Mycobacterium tuberculosis, the causative agent of TB, imposes a substantial burden on both health and economies, particularly in developing nations, such as those in Asia and Africa [7,8]. India, notably, bears the highest burden of TB globally, with an estimated 2.64 million cases in 2019 [9]. Despite the Indian government’s provision of free diagnostic and medical services for registered TB patients, research highlights the substantial financial strain and potential catastrophic costs associated with TB [10].
Furthermore, individuals with TB, especially those with pulmonary cases, have been observed to have associations with various Candida species [11]. Patients who are on immunosuppressive medications or have a history of such treatments are particularly vulnerable to Candida infections. Candida consists of approximately 200 species, with the most frequently implicated in clinical infections being C. albicans, glabrata, tropicalis, stellatoidea, parapsilosis, ciferri, guilliermondii, haemulonii, kefyr, and krusei [12]. The primary culprits in candidiasis are typically five prevalent species: Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, and Candida krusei [13].
The treatment of candidiasis commonly involves pharmaceuticals such as amphotericin B and fluconazole, which are classified as polyenes and azoles, respectively [14]. Candida species exhibit varying susceptibilities to antifungal agents, with some, like C. krusei and C. glabrata, displaying resistance or reduced sensitivity to fluconazole [15]. This underscores the importance of accurately identifying and testing susceptibility to select the most effective antifungal treatment [16]. Moreover, it is worth noting that even individuals with robust immune systems can experience invasive candidiasis.
This is especially relevant in the context of TB, as it compromises the immune response, heightening susceptibility to fungal infections [17]. Consequently, studying the types of Candida species and their antifungal susceptibility in clinical isolates has become crucial for managing Candida infections. While numerous studies worldwide, including various regions of India, have investigated the speciation and antifungal susceptibility of Candida isolates [18,19], there is a notable gap in research examining the prevalence of Candida species across all hospital wards and specimen types in a tertiary care center. Additionally, more rigorous and systematic investigations are needed to assess the prevalence of pulmonary TB among patients in the Union Territory of Puducherry. The aim of this study is to isolate and identify Candida albicans from TB patients and determine its antifungal susceptibility.
Materials and Methods
The present cross-sectional study was conducted at Aarupadai Veedu Medical College and Hospital in Puducherry, India, specifically in the Department of Microbiology and the Department of Chest Medicine. Permission from the Institutional Human Ethical Committee (IEC) was obtained. Data collection took place between August 2016 and January 2018. Informed written consent was obtained from the subjects who participated in the study, with individuals under the age of majority requiring parental or legal guardian authorisation after receiving appropriate information.
Inclusion criteria: Patients aged 21 to 60 years, all genders, including transgender individuals, known and previous TB patients were included in the study.
Exclusion criteria: Patients under antifungal therapy, patients undergoing chemotherapy for malignancy, patients who refused to participate in the study were excluded from the study.
Sample size estimation: The sample size for the study was determined to be 129 based on a 95% confidence interval, a margin of error of 4%, a Z-score of 1.96, and a prevalence rate of 4.03% [20], using the following formula:
The formula
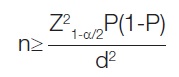
can be utilised to derive the sample size (n). In this context, the variables are defined as follows: Z represents the Z-score, P represents the prevalence rate, Q corresponds to one minus the prevalence rate, and d represents the margin of error.
Study Procedure
The focus of the research was on patients hospitalised in the chest medicine ward who exhibited symptoms such as cough, fever, dyspnoea, and expectoration. Before collecting sputum samples, complete information about the study was provided to the patients at AVMC&H, and specific consent was obtained from each patient. A total of 129 patients suspected of having pulmonary TB were analysed (101 males and 28 females) in age groups ranging from 21 to 60 years during the study period.
Sample collection: The collected sputum samples were processed using Gram stain, Ziehl-Neelsen stain, KOH mount, and cultured on blood agar and MacConkey agar. To confirm TB in patients, sputum samples were examined for acid-fast tubercle bacilli. Further investigations were conducted on the 129 suspected TB patients who were also suspected of having fungal infections. Positive samples underwent Gram staining and microscopic examination to confirm these suspected fungal infections. Under the microscope, the presence of yeast cells and filaments consistent with fungal morphology provided evidence of co-existing fungal infections in these TB patients, characteristic of fungal morphology.
Isolation and identification of C. albicans: Clinical samples underwent cultured on Sabouraud Dextrose Agar (SDA) and incubated at both 25°C and 37°C. These cultures were examined daily for microbial growth and subsequently subcultured on SDA. The subcultures were incubated at 37°C for 24 hours. On SDA, Candida species produced creamy, smooth, pasty, and convex colonies within 24 to 72 hours. Some species required more than three days to become visible on the culture medium. The isolated colonies obtained on SDA were further subjected to Gram staining to identify budding yeast cells and pseudohyphae. Conventional methods, including the Germ tube test, demonstration of chlamydospore formation on Cornmeal agar with Tween 80, sugar fermentation tests, and sugar assimilation tests, were employed for speciation [21].
Candida isolates were subcultured on HI Chrome agar plates for further identification and incubated at 37°C for 48 hours. The colonies were identified based on the colour produced according to the manufacturer’s instructions [22], resulting in the following identifications:
Candida albicans: Light green-coloured smooth colonies.
Candida tropicalis: Blue to metallic blue-coloured raised colonies.
Candida glabrata: Cream to white smooth colonies.
Candida krusei: Purple fuzzy colonies.
Candida parapsilosis: Light pink colonies.
Antifungal susceptibility testing: Antifungal susceptibility testing was performed using the disk diffusion method according to the CLSI (formerly NCCLS) 2009 M44-A2 guidelines. Commercially available 6 mm antifungal discs (Himedia, Mumbai, India), including fluconazole (25 μg), voriconazole (1 μg), amphotericin B (20 μg), itraconazole (10 μg), and ketoconazole (30 μg), were used [23]. Mueller-Hinton agar supplemented with 2% glucose and 0.5 mg/L methylene blue was utilised as the medium. The inoculum was taken from the SDA tubes using a sterile swab and standardised to a 0.5 McFarland turbidity. Susceptibility testing was performed on Mueller-Hinton agar plates with 2% glucose and 0.5% methylene blue. Antifungal drug discs, including itraconazole, voriconazole, fluconazole, ketoconazole, and amphotericin B, were applied and then incubated at 37°C for 24 to 48 hours [24].
Statistical Analysis
The data collected was entered into a Microsoft Excel sheet. Descriptive statistics were used to analyse the data. The Chi-square test was applied, and a p-value of <0.05 was considered significant for the analytical statistics.
Results
Out of the 129 patients in the study, 101 (78.29%) were males, and 28 (21.71%) were females. A higher incidence of 45 (34.88%) was observed in the age group of >60, with the highest number of patients in the age group of 41-60 years, totaling 60 (46.51%). The least number of patients was found in the age group of 21-40 years, with 24 (18.61%) patients [Table/Fig-1].
Age-wise prevalence of pulmonary Tuberculosis (TB).
| Age group (years) | Male | Female | n (%) |
|---|
| n (%) | n (%) |
|---|
| 21-40 | 17 (13.18) | 7 (5.43) | 24 (18.61) |
| 41-60 | 47 (36.43) | 13 (10.08) | 60 (46.51) |
| >60 | 37 (28.68) | 8 (6.2) | 45 (34.88) |
| Total | 101 (78.29) | 28 (21.71) | 129 (100) |
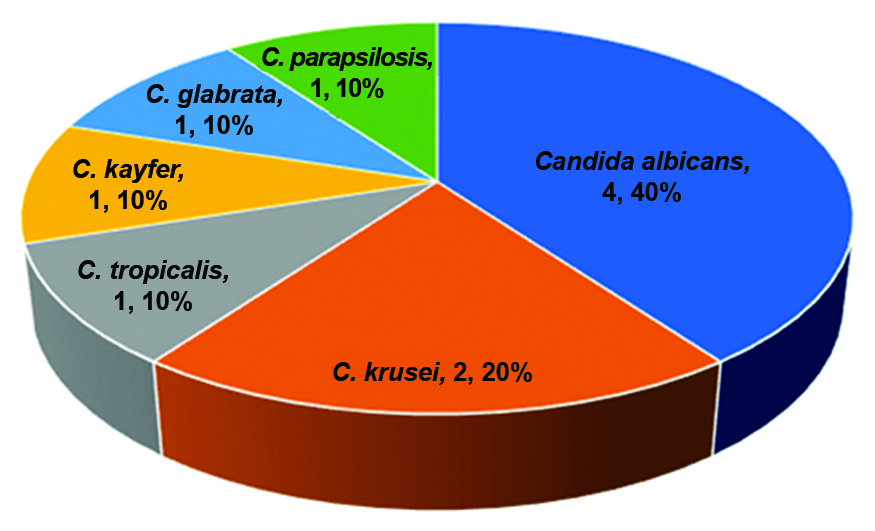
Distribution of Candida spp.: Out of the 129 patients, 10 (7.75%) had Candida species distribution is shown in [Table/Fig-2].
Distribution percentage of Candida in pulmonary Tuberculosis (TB) patients.
Positivity among the sputum samples was determined based on AFB (Acid-Fast Bacilli) testing. Out of the 129 patients from whom sputum samples were obtained, 71 (55.04%) purulent samples tested positive for AFB, while 25 (19.38%) tested negative. Among the mucopurulent samples, 19 (14.73%) were AFB-positive, with 8 (6.20%) testing negative. For the mucoid sputum samples, 2 (1.55%) were AFB-positive, while 4 (3.10%) were AFB-negative. The nature and classification of these sputum samples are shown in [Table/Fig-3]. The result was not significant (p-value <0.05).
Nature of clinical sputum samples.
| Nature ofsputum | n (%) | Positiven (%) | Negativen (%) | Chi-squaretest | p-value |
|---|
| Purulent | 96 (74.42) | 71 (55.04) | 25 (19.38) | 4.571 | 0.107 |
| Muco purulent | 27 (20.93) | 19 (14.73) | 8 (6.20) |
| Mucoid | 6 (4.65) | 2 (1.55) | 4 (3.10) |
Chi-square test applied; Significant (p<0.05)
Out of 129 patients, 5 (3.87%) had Aspergillus species. A. flavus was isolated in 1 (0.78%) of the patients whose sputum was AFB positive. A. fumigatus was isolated in 2 (1.56%) of the patients, 1 (0.78%) with sputum positive and the other one (0.78%) with sputum negative. A. niger was isolated in 2 (1.56%) of the patients, with 1 (0.78%) being sputum positive and the other sputum negative [Table/Fig-4]. Aspergillus species were not the primary focus of the study, but this additional observation was made and documented here.
Detection of Aspergillus spp in pulmonary Tuberculosis (TB) patients.
| Species | Identifiedn (%) | Positiven (%) | Negativen (%) |
|---|
| A.flavus | 1 (0.78) | 1 (0.78) | 0 |
| A.fumigatus | 2 (1.56) | 1 (0.78) | 1 (0.78) |
| A.niger | 2 (1.56) | 1 (0.78) | 1 (0.78) |
Antifungal efficacy: The antifungal efficacy was tested against various species and antifungals, including fluconazole, voriconazole, itraconazole, ketoconazole, and amphotericin B [Table/Fig-5]. The maximum sensitivity of 4 (100%) was observed in C. albicans when treated with itraconazole and amphotericin B, followed by 3 (75%) for ketoconazole, and 2 (50%) each for fluconazole and voriconazole. The highest percentage of sensitivity to Candida was seen with itraconazole and amphotericin B. Notably, C. tropicalis and C. kyfer showed 0 sensitivity to fluconazole and voriconazole. The maximum sensitivity was observed against C. krusei, followed by C. glabrata and C. parapsilosis.
Results of Antifungal Susceptibility Testing (AST) conducted on isolated fungi.
| Antifungal drugs |
|---|
| Species | Fluconazole | Voriconazole | Itraconazole | Ketoconazole | Amphotercin B |
|---|
| n (%) | n (%) | n (%) | n (%) | n (%) |
|---|
| C. albicans | 2 (50%) | 2 (50%) | 4 (100%) | 3 (75%) | 4 (100%) |
| C. tropicalis | 0 | 0 | 1 (100%) | 1 (100%) | 1 (100%) |
| C. krusei | 2 (100%) | 2 (100%) | 2 (100%) | 2 (100%) | 2 (100%) |
| C. kyfer | 0 | 0 | 1 (100%) | 0 | 1 (100%) |
| C. glabrata | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) |
| C. parapsilosis | 1 (100%) | 0 | 1 (100%) | 0 | 1 (100%) |
Discussion
As a global issue with a changing prevalence and incidence rate, TB is a serious health problem [25]. In recent times, there has been an increase in the prevalence of opportunistic fungal infections, which do not typically become pathogenic in normal, healthy individuals [26]. These infections can become pathogenic in people with compromised immunity due to underlying diseases, increased consumption of broad-spectrum antibiotics, or those who have pulmonary TB. When pulmonary TB is co-infected with a fungal infection, morbidity is increased [27].
Males were more likely to have pulmonary TB, and their rates of co-infection with fungi were higher than those of females in the present study. This may be because of the increased exposure to the external environment among males, as they often work outside. In addition, smoking is more prevalent in men, and in developing countries, men generally have greater access to healthcare facilities [28].
The most common age group for pulmonary fungal infection with Aspergillus is middle-aged, as opposed to the elderly, and males are more likely to be infected [29]. Although the detection of Aspergillus species was not the primary aim of the study, it was an additional observation made during the research process. Including the results related to Aspergillus might be relevant for providing a comprehensive overview of fungal infections in the studied population, even though the primary focus was on Candida.
The current study’s outcomes align with those of Kohno S et al., and other researchers, affirming that individuals in their third and fourth decades have a higher prevalence of fungal mycosis compared to younger people [30]. Men within these age brackets exhibited more cases than women. Previous studies have also linked fungal infections mostly to individuals in their 30’s and 40’s [31,32].
According to these findings, age seems to influence the occurrence of fungal infections. Children under 10 years old did not exhibit any systemic infections, while those over 60 had a low incidence rate. Conversely, patients in middle age presented a high prevalence, possibly due to greater environmental exposure and secondary infections. The most frequently diagnosed Aspergillus species, such as A. fumigatus, A. flavus, and A. niger, were identified, along with Candida albicans, from sputum samples collected from TB cases in the current study, affirming Osman NM et al.’s results, which showed A. fumigatus as the most commonly observed isolate among Aspergillus species in their analysis of 50 TB cases [33]. Additionally, Ekenna O et al., confirmed TB [34]; however, the present research demonstrated some variation, as the predominant isolates were A. fumigatus, compared to Razmpa et al.’s findings, which reported a higher presence of A. flavus during sample collection [35].
Kashid RA et al., reported a prevalence of 13.33% for C. albicans [36]. In the present study, Candida albicans was the most common fungal species, causing co-infection in four cases (40%). This was followed by C. krusei in two cases (20%), C. tropicalis in one case (10%), C. kefyr in one case (10%), C. glabrata in one case (10%), and C. parapsilosis in one case (10%). The prevalence of Candida was higher in men compared to women, which was consistent with the findings of Roy RC et al., who reported C. tropicalis as the predominant species in HIV-positive patients [37]. Similar studies conducted by Bhavana C and Singh R et al., have reported Candida tropicalis as the major isolate, ranging between 26-59% [38,39]. A study carried out by Khadka S et al., found that Candida tropicalis is the major isolate among NAC species, followed by Candida glabrata, which was comparable to the present study [40]. These findings suggest that NAC species are becoming increasingly important pathogens, gradually replacing Candida albicans over the past 2-3 decades [41]. In contrast to these findings, the present study showed Aspergillus species as the most important fungi. The observations of the present study were consistent with previous studies by other authors, who also found a significant predominance of Candida albicans [Table/Fig-6] [17,19,42-48].
Comparison of sensitivity patterns of Candida species to antifungals among various studies [17,19,42-48].
| Authors with places and years of publication | Jaycharan AL et al., Chennai, 2018 (%) [17] | Bhaskaran R et al., Thrissur, 2020 (%) [19] | Talukdar A et al., Guwahati, 2020 (%) [42] | Shukla R et al., Telangana, 2020 (%) [43] | Deepthi KN et al., Kerala, 2020 (%) [44] | Shwetha DC and Venkatesha DC, Karnataka, 2021 (%) [45] | Patil and Nerurkar E, Mumbai, 2021 (%) [46] | Chen J et al., China, 2021 (%) [47] | Chongtham U Manipur, 2022 (%) [48] | Present study, Puducherry 2024 (%) |
|---|
| Candida spp. with antifungals |
|---|
| C. albicans | F | 88.2 | 100 | 85 | 80 | 96.7 | 85.1 | 77 | 92.5 | 79.5 | 50 |
| V | - | 100 | 100 | 88 | 100 | 85.1 | 78 | 92.5 | 84.1 | 50 |
| It | 89.2 | - | - | - | - | - | 94 | - | 79.5 | 100 |
| Kt | - | - | - | - | - | - | - | - | 63.6 | 75 |
| AmpB | 98.9 | 96 | 100 | 84 | - | 100 | 97.3 | - | 93.2 | 100 |
| C. tropicalis | F | 83.3 | 100 | 28.5 | 65.2 | 91.5 | 93.8 | 79 | 80.9 | 31.2 | 0 |
| V | - | 100 | 85.7 | 82.6 | 100 | 96.9 | 79 | 61.9 | 81.3 | 0 |
| It | 76.6 | - | - | - | - | - | 93.5 | - | 56.2 | 100 |
| Kt | - | - | - | - | - | - | - | - | 50 | 100 |
| AmpB | 96.6 | 100 | 71.4 | 89.1 | - | 100 | 94.5 | - | 65.6 | 100 |
| C. krusei | F | 0 | 0 | 28 | 0 | 0 | 0 | - | - | 0 | 100 |
| V | - | 100 | 28 | 100 | 83.3 | 66.7 | 100 | - | 100 | 100 |
| It | 72.7 | - | - | - | - | - | - | - | 40 | 100 |
| Kt | - | - | - | - | - | - | - | - | 40 | 100 |
| AmpB | 100 | 0 | 64 | 100 | - | 100 | 66 | - | 60 | 100 |
| C. kyfer | F | - | - | - | - | - | - | - | - | - | 0 |
| V | - | - | - | - | - | - | - | - | - | 0 |
| It | - | - | - | - | - | - | - | - | - | 100 |
| Kt | - | - | - | - | - | - | - | - | - | 0 |
| AmpB | - | - | - | - | - | - | - | - | - | 100 |
| C. parapsilosis | F | 100 | 100 | - | 100 | 87.5 | - | 60 | 91.9 | 50 | 100 |
| V | - | 92.3 | - | 100 | 100 | - | 80 | 94.6 | 100 | 0 |
| It | 100 | - | - | - | - | - | - | - | 25 | 100 |
| Kt | - | - | - | - | - | - | - | - | 0 | 0 |
| AmpB | 100 | 100 | - | 50 | - | - | 95 | - | 50 | 100 |
F: Fluconazole, V: Voriconazole, It: Itraconazole, Kt: Ketoconazole, AmpB: Amphotericin B
Antifungal susceptibility testing was performed in the study. The antifungal drugs used were fluconazole, voriconazole, itraconazole, ketoconazole, and amphotericin B. Candida species were identified using chrome agar for all Candida species. The results of the present study showed a total of 10 (7.75%) Candida isolates, including C. albicans (4), C. tropicalis (1), C. krusei (2), C. kefyr (1), C. glabrata (1), and C. parapsilosis (1). C. albicans showed higher sensitivity to itraconazole and amphotericin B, followed by ketoconazole, fluconazole, and voriconazole. C. krusei and C. glabrata exhibited 100% sensitivity to all antifungal drugs used. Therefore, the antifungals itraconazole and amphotericin B, which are typically 100% sensitive to most Candida species, are listed and correlated with similar sensitivity patterns observed in other studies, as depicted in [Table/Fig-6] [17,19,42-48].
Males are more likely to develop the condition due to their increased exposure to the outside environment and propensity for utilising various addictive substances. However, it is important to note that the findings of this study might not be representative due to the limited study group; therefore, it must be verified through further research. From a clinical standpoint, these findings underscore the importance of personalised treatment for Candida infections, especially for C. albicans. Itraconazole and amphotericin B are potential first-line options, considering the potential resistance to other antifungals. Future research should aim to expand sample sizes, delve into genetic factors impacting drug sensitivity, and consider regional variations to enhance tailored antifungal therapies. These efforts will form a foundation for ongoing optimisation of Candida infection treatment.
Limitation(s)
Sputum samples were used for testing, which may not be the most reliable method for determining the specific type of pulmonary TB infection a person has. Additionally, there is a need to study the increase in Minimum Inhibitory Concentration (MIC) values over time among different Candida isolates in specific geographic locations.
Conclusion(s)
Candida albicans has been found to be the most common co-infection in TB patients. Present research highlights the increasing threat posed by NAC, some of which are resistant to azole antifungals. The prevalence of Candida species is on the rise in tertiary care hospitals, particularly among patients with pulmonary TB who also have Candida albicans infections. Despite receiving antitubercular therapy, a majority of male patients continued to experience pulmonary symptoms.
It is recommended to incorporate epidemiological data on fungi and routine antifungal susceptibility testing for Candida in the management of pulmonary TB patients. The findings led us to conclude that drug-resistant Candida strains are frequently present in the sputum and respiratory tracts of individuals with respiratory tract infections. Therefore, regular screening for Candida infection should be conducted in pulmonary TB patients, along with antifungal sensitivity testing for NAC. Additionally, prompt therapy, effective treatment, and species identification are crucial for reducing patient morbidity and mortality.
Chi-square test applied; Significant (p<0.05)
F: Fluconazole, V: Voriconazole, It: Itraconazole, Kt: Ketoconazole, AmpB: Amphotericin B