The treatment of acute and terminal irreversible liver conditions frequently involves LTx. In 2021, 34,694 liver transplants were performed globally, an increase of 6.5% from 2020 and 20% from 2015 [1]. Post-transplant liver allograft biopsies are considered the gold standard for managing terminal conditions. Despite the widespread use of biochemical tests, these tests usually fail to distinguish between rejection and other potential diseases that could manifest in the allograft [2]. These biopsies play a crucial role in identifying the underlying cause of changes in liver function that induce hepatocellular or biliary damage [3].
Long-term IMS, complex clinical circumstances, and de novo complications that overlap with the histologic traits of various pathologies are the primary contributors to the critical challenges of transplant pathology. Therefore, LTx requires a multidisciplinary approach, and pathologists can significantly contribute at every stage of the process. Pathologists are increasingly engaged in evaluating graft quality and function both before and after LTx. Despite the importance of this role, similar studies are rare in this demographic.
As a major tertiary care centre in Southern India, the aim was to share experiences regarding the reasons for and outcomes of post-LTx biopsies at our facility. The objective was to study the histopathologic assessment of allograft liver biopsies in differential diagnosis, timing, and prevalence of post-transplantation complications, including identifying the causes of graft damage. Given the diverse management strategies that may be warranted, the accurate identification of prevailing challenges and the interpretation of results in a proper clinical and therapeutic context is critical.
Materials and Methods
This retrospective cross-sectional research study was conducted between January 2019 and June 2022 at a major tertiary care centre in Southern India. All transplants were performed at VPS Lakeshore Hospital in Kochi, Kerala, India. This study did not require approval from the Institutional Review Board of our institution owing to its retrospective nature. However, informed consent was obtained from all patients at the time of the procedure. Most of transplants performed were orthotopic transplants, split donations from cadaveric donors, or living donor transplants, which constituted 90% of the transplants. All transplants were conducted according to the ethical standards set forth by the responsible committee on human experimentation (institutional or regional) and adhered to the principles of the Helsinki Declaration of 1975, as revised in 2013.
Inclusion criteria: Patients who underwent transplantation at the facility and subsequently developed postoperative complications during this study period were included in the study.
Exclusion criteria: Post-transplant recipients whose course was uneventful during the study period were excluded from the study.
Study Procedure
The histopathology lab received 45 post-transplant needle biopsy specimens. The first biopsy was taken shortly after transplantation, and the last was performed 770 days later. All patients, both paediatric and adult, who had transplants during the 3.5-year study period were included. Cases for evaluation in which paraffin blocks were unavailable were excluded from this study.
Eight consecutive sections, each 3 mm thick, were cut from paraffin-embedded biopsies after the samples were fixed in 10% neutral buffered formalin. The biopsy sections were stained using Haematoxylin and Eosin (H&E), Masson’s trichrome, reticulin, Periodic Acid-Schiff (PAS) with and without diastase, and Prussian blue. Immunohistochemical analysis was performed as indicated, including stains for cytokeratin 7, Cytomegalovirus (CMV), HSV types 1 and 2, and C4d (wherever indicated). For example, if CMV infection was suspected, immunohistochemistry for CMV antigen was conducted. If chronic rejection was suspected, immunohistochemistry for CK7 was performed to identify bile ducts.
The evaluation of antibody-mediated rejection was based on the criteria proposed by the Banff schema for grading liver allograft rejection, specifically using the C4d immune score for formalin-fixed, paraffin-embedded samples. The scoring system is as follows:
Score (0): No C4d deposition in portal microvasculature.
Score (1): Minimal (<10% portal tracts) C4d deposition in >50% of the circumference of portal microvascular endothelia (portal veins and capillaries).
Score (2): Focal (10-50% portal tracts) C4d deposition in >50% of the circumference of portal microvascular endothelia (portal veins and capillaries), usually without extension into periportal sinusoids.
Score (3): Diffuse (>50% portal tracts) C4d deposition in >50% of the circumference of portal microvascular endothelia (portal veins and capillaries), often with extension into inlet venules or periportal sinusoids.
Clinical data, including age, sex, pre-and post-transplant clinical facts (including the cause of the transplant and pathological diagnosis), were extracted from the hospital data system and the central laboratory information management system. To provide correct histological opinions, the clinical data included biopsy timing, the initial rationale for the transplant, and information about biochemical and serological parameters, as well as the immunosuppressive status during the biopsy. The slides were retrieved from histopathology storage and reviewed by two pathologists.
Similarly, following characteristics were examined in liver biopsies: portal tract count and biopsy adequacy; lobular architecture; the presence of pathological conditions such as cholestasis, biliary obstruction, sepsis, and acute and chronic graft rejection; recurrence of underlying conditions; drug toxicity; surgical complications; opportunistic infections; and de novo diseases.
The Banff Working Group’s 2016 Update was utilised to grade acute and chronic rejection [4].
Statistical Analysis
The analysis was done on the collected data and shown in the form of number and percentages.
Results
Among 84 liver allograft recipients during the study period, 38 patients developed complications in the postoperative period and were subjected to 45 needle biopsies. The remaining 46 patients had an uneventful postoperative course. One, five, and 32 patients underwent triple, double, and single biopsies, respectively. Among the 38 participants, 29 were men and 9 were women. The ages of the patients ages spanned from 8 to 70 years. The major indications for LTx are largely consistent with the most recent practice recommendations by the American Association for the Study of Liver Disease [Table/Fig-1] [5].
Common indications of Liver Transplant.
| Primary aetiology | Number of cases (n=38) | Percentage (%) |
|---|
| Decompensated CLD | 33 | 86.84 |
| -HCV- related | 2 | 6.06 |
| -HBV- related | 2 | 6.06 |
| -Alcoholic liver disease | 1 | 3.03 |
| -Non alcoholic steatohepatitis | 2 | 6.06 |
| -Arsenic exposure | 1 | 3.03 |
| -Cryptogenic | 25 | 75.75 |
| Primary hepatic neoplasm | 2 | 5.26 |
| -HCC |
| Acute liver failure | 1 | 2.63 |
| Cholestatic liver disease | 1 | 2.63 |
| -Biliary atresia |
| Inherited metabolic liver diseases | 1 | 2.63 |
| -Wilson’s disease |
CLD: Chronic liver disease; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus;
HBV: Hepatitis B virus
[Table/Fig-2] presents the various histopathological lesions. In this study, the earliest biopsy was performed shortly after transplantation, while the latest was performed after 770 days post-transplantation. TCMR was the most common pathology observed between 12 days and 11 months after transplantation, affecting nine patients. The degree of TCMR ranged from mild in three biopsies (33.33%) to moderate in five (55.55%) and severe in one (11.11%) [Table/Fig-3].
Distribution of post-transplant liver biopsy cases.
| S. No. | Diagnosticaetiology | Number of cases (n=45) | Percentage (%) | Timingpostoperative day |
|---|
| 1. | T-Cell-Mediated Rejection (TCMR) | 9 | 20.00 | |
| -Mild (RAI=3,4) | 3 | 33.33 | 12 days to 11 months |
| -Moderate (RAI=5,6) | 5 | 55.56 | 8 days to 4 months |
| -Severe (RAI ≤6) | 1 | 11.11 | 1 month 4 days |
| 2. | IHC | 11 | 24.44 | 1 day to 2 years |
| -Sepsis | 4 | 36.4 | 7 days to 18 days |
| -Drug-induced cholestatic hepatitis | 3 | 27.2 | 20 days to 1.2 years |
| Unknown | 4 | 36.4 | |
| 3. | Biliary obstruction | 5 | 11.11 | 1 day to 20 days |
| 4. | HSV | 3 | 6.67 | 11 days to 6 months |
| 5. | Plasma Cell-Rich Rejection (PCRR) | 2 | 4.44 | 40 days to 7 months |
| 6. | ICP | 2 | 4.44 | 7- 8 months |
| 7. | DHF | 2 | 4.44 | 2 days- 7 days |
| 8. | Veno occlusive-like disease | 2 | 4.44 | 6 months to 7 months |
| 9. | Ethanol-induced hepatic injury | 1 | 2.22 | 7 months |
| 10. | PRI | 1 | 2.22 | 12 days |
| 11. | AMR | 1 | 2.22 | 10 days |
| 12. | Vascular neoplasm | 1 | 2.22 | 8 months |
| 13. | Normal pathology | 5 | 11.11 | 7 months to 2 years |
RAI: Rejection activity index; IHC: Intrahepatic cholestasis; ICP: Isolated central perivenulitis; HSV: Herpes simplex virus; DHF: Dengue haemorrhagic fever; PRI: Preservation reperfusion injury; AMR: Antibody-mediated rejection
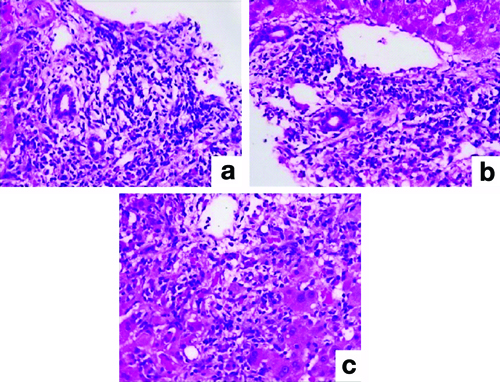
Severe T-Cell-Mediated Rejection (TCMR): (a) Mixed portal inflammatory infiltrate (H&E; 40x); (b) Endothelitis (H&E; 200x); (c) Central venulitis (H&E; 100x).
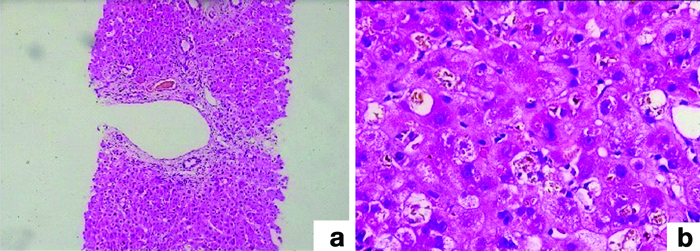
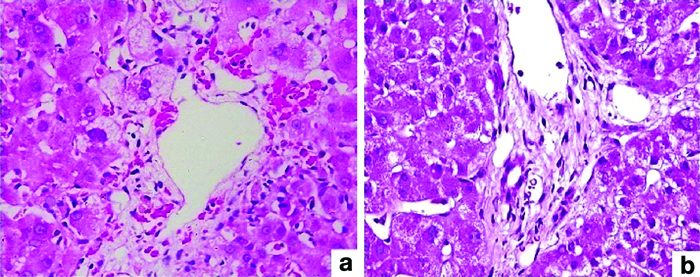
In 24.44% (11 of 45) of the cases, Intrahepatic Cholestatis (IHC) was detected during the biopsy, and the patients’ bilirubin levels ranged from 3.9 to 28.6 mg/dL. A biopsy was performed in these cases from 1 day to 2 years after transplantation. In four biopsies (8.88%) performed 18 days after transplantation, sepsis was detected as the cause of IHC. Within a time range of 1-20 days, five (11.11%) samples showed significant bile duct obstruction [Table/Fig-4], resulting in increased bilirubin levels. Between 11 days and six months after transplantation, Herpes Simplex Virus (HSV) infection was noted in three biopsies (6.66%) [Table/Fig-5].
(b) Ductular reaction and ductular cholestasis (H&E; 200x); (a) Feathery degeneration and canalicular cholestasis (H&E; 40x).
Pathologic features of Herpes Simplex Virus (HSV): (a) Parenchyma riddled with multiple, irregular, and randomly distributed foci of coagulative necrosis (H&E; 40x); (b) Ground glass inclusions (H&E; 100x); (c) Immunohistochemistry, HSV1 (IHC; 100x).
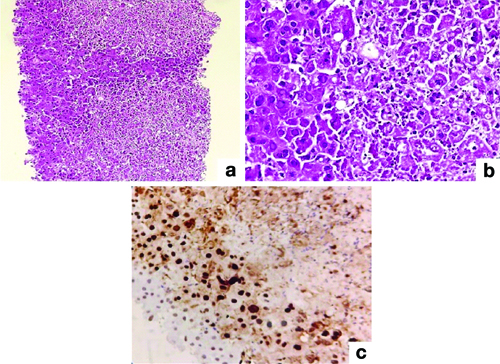
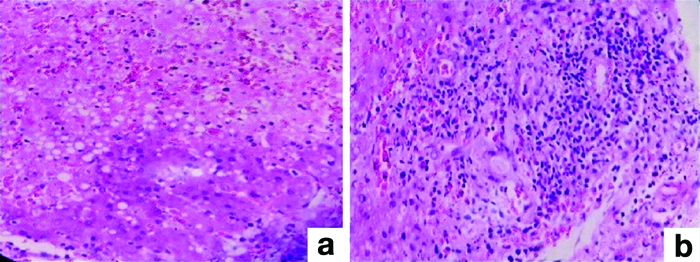
Immunohistochemistry was used to confirm HSV cases using HSV1 immunohistochemistry, two antigens, and serum PCR. Two patients with Chronic Liver Disease (CLD) as their underlying condition developed PCRR and central perivenulitis [Table/Fig-6]. Central perivenular necrosis with portal tract changes and veno-occlusive-like disease suggestive of late acute TCMR was observed in 2 (4.44%) patients who were 6-7 months post-transplant. In two cases, perivenular hepatocyte dropout and necrosis indicated ICP. There was no associated portal tract alterations in these patients [Table/Fig-7].
Pathologic features of Plasma Cell-Rich Rejection (PCRR): (a) Central venulitis (H&E; 100x); (b) Plasma cell rich infiltrate (>30%) (H&E; 100x); (c) Perivenular sclerosis (Masson trichrome; 200x).
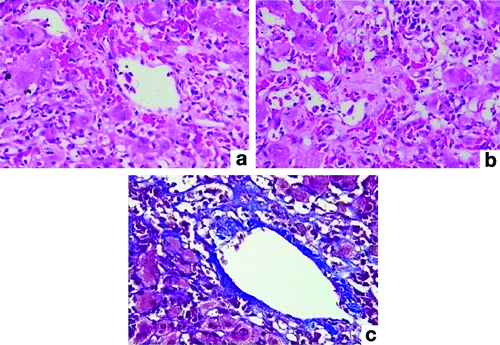
Pathology of isolated central perivenulitis; (a) Perivenular hepatocyte dropout (H&E; 100x); (b) Portal tract showing no histologic changes (H&E; 100x).
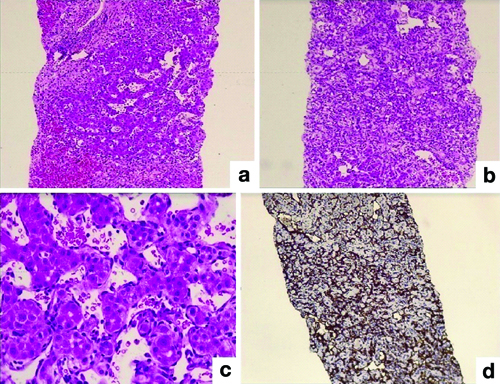
On the 10th and 12th days after transplantation, one patient each showed Antibody-Mediated Rejection (AMR) and Preservation Reperfusion Injury (PRI). Furthermore, eight months after the transplant, recurrent vascular neoplasm due to arsenic exposure was observed in one case [Table/Fig-8]. Other unusual diagnosis included ethanol-induced hepatic injury and infections such as Dengue Haemorrhagic Fever (DHF) [Table/Fig-9].
Pathology of recurrent vascular neoplasm: (a) Vascular neoplasm (H&E; 100x); (b) Hepatocyte atrophy and sclero hyalinisation (H&E; 40x); (c) Interconnecting vascular channels (H&E; 200x); (d) Immunohistochemistry, CD31 (IHC; 40x).
Pathology of Dengue haemorrhagic fever: (a) Haemorrhagic necrosis (H&E; 100x); (b) Portal tract expansion by moderate infiltrates of lymphocytes and few eosinophils (H&E; 100x).
Discussion
This study provides a comprehensive analysis of critical complications following LTx, shedding light on the types, frequency, and timing of these complications. Post-transplant liver biopsies, typically termed PTLBs, are essential for treating transplant patients and offer crucial information regarding disease rejection or recurrence. Many centres conduct protocol biopsies at 0, 1, and 6 months to identify graft dysfunction and to anticipate it at an early stage. The role of modern surgical pathologists includes interpreting PTLBs, which can have various therapeutic effects.
It is crucial to consider various potential causes of allograft injury after transplantation, not just rejection, and to factor in the time elapsed since the transplant. Functional cholestasis can persist for several weeks post-transplant and should be differentiated from cholestasis caused by acute rejection, bile duct obstruction, drugs, or sepsis when possible. Early postoperative cholestasis might also result from preservation reperfusion injury or a “small-for-size” graft. During the first year after transplantation, it is important to consider the recurrence of pre-existing conditions, such as autoimmune hepatitis or hepatitis B and C. Chronic rejection typically manifests later, generally between 6 to 12 months post-transplant. By taking into account the timeline of the transplant, along with clinical and laboratory findings, it becomes possible to narrow down the potential diagnosis (as detailed in [Table/Fig-10]). Analysing histologic patterns can then provide additional insights to distinguish between these conditions and achieve a definitive diagnosis.
Differential diagnosis based on the post-transplant timeline.
| Time frame | Differential diagnosis |
|---|
| Immediate post-transplant period | Preservation reperfusion injury |
| Vascular obstruction |
| Antibody mediated |
| 0-1 month | Acute rejection |
| Preservation reperfusion injury |
| 1-12 months | Acute rejection |
| Surgical complications (Ischaemia, Biliary complication) |
| Infection |
| Recurrent disease |
| >12 months | Chronic rejection |
| Recurrent disease |
| Late acute cellular rejection |
| Biliary complication from surgery |
| Infection |
Despite the liver’s tolerogenic immunological milieu and efficient immune system mechanisms that decrease the graft-directed immune response, allograft rejection remains a risk in LTx. As a result, identifying early signs of rejection is critical for future therapeutic measures and for preventing undesirable consequences such as graft loss. The three primary rejection patterns are hyperacute, acute, and chronic, and their pathophysiological causes, clinical characteristics, and onset times vary [6].
The leading cause of early graft dysfunction is TCMR, previously referred to as acute cellular rejection, which occurs in 24-80% of cases. The major characteristics of this portal-based inflammatory process include endotheliitis, bile duct damage, and heterogeneous infiltrates of inflammatory cells. The Banff Working Group [4] proposes diagnostic and grading criteria that provide a comprehensive evaluation and a more particular semiquantitative index, the Rejection Activity Index (RAI), for assessing the degrees of engagement of the targeted structures. The RAI is higher when there is more inflammation and involvement of anatomical tissues. There are three rejection levels: indeterminate (1,2); mild (3,4); moderate (5,6); and severe (>6).
In present study, acute TCMR, was noted in 20.00 % of cases. Most of these cases were classified as moderate acute rejections, with a Rejection Activity Index (RAI) of 5 or 6. This finding was consistent with earlier investigations [7]. Authors from India reported acute TCMR as the most common post-transplant pathology in 33.3% of cases [8].
Significant differential diagnosis for rejection include Preservation Reperfusion Injury (PRI), Hepatitis C Virus (HCV) recurrence, Epstein-Barr Virus (EBV) infection, drug toxicity, and postoperative complications. Regarding late T-cell-mediated rejection (Late Acute Rejection, LAR), some authors [9] have reported that acute rejection that manifests six months after transplantation is referred to as LAR. This reaction appears to “tone down” over time after transplantation and lacks the classic features of TCMR previously observed. Histological characteristics of LAR include predominantly mononuclear portal inflammatory infiltration, less notable venous endothelial injury, lymphocytic bile duct damage, perivenular necroinflammatory-type activity, and alterations resembling those seen in Veno-Occlusive Disease (VOD) [10]. Present study observed this in two cases where a biopsy revealed perivenular hepatocyte dropout and necrosis, indicative of central perivenulitis. Corresponding alterations included VOD and associated portal tract inflammation.
Regarding biliary complications, post-transplant cholestasis may be intrahepatic or extrahepatic and can involve mechanical obstruction of the main bile ducts. Approximately 10-20% of patients experience biliary complications after LTx, which remain a prominent cause of post-transplant morbidity. Complications following bile duct surgery may include stones, casts, biliary strictures, and bile leakage. In addition to elevated liver function tests, patients with cholestasis and/or cholangitis may experience fever and right-sided abdominal pain. Available treatments include surgical reconstruction, stenting, and endoscopic dilatation. Histological features of biliary obstruction include expanded portal tracts caused by a ductular reaction, accompanied by neutrophils, ductal, ductular, and canalicular cholestasis, as well as intrahepatic cholestatis with feathery degeneration. Present study discovered bile duct obstruction in 11.11% (5 of 45) of PTLBs.
IHC was detected in 24.44% (11 of 45) of the cases. Biopsy results revealed perivenular to panlobular cholestasis without considerable portal inflammation, ductulitis, endotheliitis, or ductular reaction, along with ballooning hepatocyte degeneration. Another cause of IHC is sepsis; 19-25% of transplant recipients experience bacteremia within 30 days, causing 22-36% of all severe infections in this patient population [11]. Patients with a superimposed infection may present with ascending cholangitis, characterised by neutrophilic infiltration of the biliary epithelium and the duct lumina, bile duct proliferation, and bile plugs.
Many drugs required for liver transplant patients can be hepatotoxic and lead to IHC. Therefore, cholestatic damage in the early and late post-transplant periods may be owing to Drug-Induced Liver Injury (DILI). Owing to the many potential causes of liver injury, diagnosing DILI in LTx cases can be challenging.
The following distinctions between the various types of cholestatic DILI can be made using histology in the differential diagnosis procedure [12]: First, in the absence of pertinent inflammation, acute pure cholestasis manifests as hepatocyte cholestasis, canalicular dilatation, and bile plugs. Second, acute cholestatic hepatitis is characterised by cholestasis, inflammation, and, in rare cases, hepatocellular necrosis. Third, cholestasis with bile duct injury occurs when there is ductular, cholangiolar, or cholangiolytic damage, but little hepatocellular injury. Finally, there is vanishing bile duct syndrome.
Although the frequency of DILI in LTx recipients has significantly been reduced owing to the optimisation of tailored immunosuppressive medication regimens, the risk persists. DILI may manifest when complications or other coexisting medical conditions need to be treated. Present study diagnosed three patients with drug-induced cholestasis. Tacrolimus-related liver damage was present in one patient, presenting as perivenular necrosis.
IHC in post-transplant liver biopsies has been evaluated by Ponziani FR et al., who classified it as early or late cholestasis, depending on whether it manifests before or after six months following the transplant [13].
Infections are majorly life-threatening during the first few months following transplantation. While bacterial and fungal infections might indirectly affect the allograft, Cytomegalovirus (CMV) and Epstein-Barr Virus (EBV) are the most common causes of non hepatotropic viral hepatitis in transplant recipients. Adenovirus, herpes simplex virus, and human herpes virus six are other viruses that can infect the allograft. Present study had three cases of Herpes Simplex Virus (HSV) hepatitis (6.67%) throughout the study, identified through liver biopsies and verified through immunohistochemistry and PCR. Biopsies showed areas of confluent necrosis, with hepatocytes showing ground-glass nuclei and multinucleation.
Dengue Fever (DF) is a rarely reported disease among patients who have undergone transplantation. Present study had two patients (4.44%) with DF following LTx, with diagnosis made via IgM titre and non structural protein 1 antigen detection. One of these patients developed DHF following the transplant and succumbed to the illness. Owing to IMS, diagnosing DF in individuals who have undergone LTx is challenging.
Regarding Plasma Cell-Rich Rejection (PCRR), previously referred to as plasma cell hepatitis or de novo autoimmune hepatitis, the Banff recommendations now clearly describe it as a type of rejection that exhibits combined features of TCMR and Antibody-Mediated Rejection (AMR), overlapping with autoimmunity and contributing to late graft loss after LTx, especially in interferon-treated hepatitis C recipients. PCRR exhibits more severe and widespread lymphocytic cholangitis, over-representation of IgG4-positive plasma cells, aggressive plasma cell-rich central perivenulitis, donor-specific antibody positivity, portal microvascular C4d deposition, and other rejection-compatible characteristics. There should be no autoimmune-related condition present at the outset. This study had two PCRR cases (4.44%); Chronic Liver Disease (CLD) was the underlying disease in both cases, and both patients had negative HCV serology. This type of injury can affect approximately 3-5% of LTx recipients [6].
Central perivenulitis is a necroinflammatory lesion affecting the central (hepatic) venules and perivenular hepatocytes. Different degrees of inflammation, endotheliitis, hepatocyte necrosis, and perivenular fibrosis are present in the lesion. It typically happens in conjunction with the characteristic portal tract symptoms of acute rejection in the early post-transplant period. However, in Late Acute Rejection (LAR), it may appear as an isolated lesion known as Isolated Central Perivenulitis (ICP), which occurs most frequently six months to one year after transplantation in approximately one-third of adult and paediatric transplant recipients [14,15]. ICP has been extensively analysed by Demetris AJ et al., who postulated that ICP serves as a bridge between LAR and chronic rejection [16]. They also proposed a grading system for this condition. In present study, there were two cases of ICP (4.44%) at 7-8 months post-transplant.
Preservation Reperfusion Injury (PRI) in the first 12 days following LTx was another major concern in one case (2.22%). PRI is common, mild, and typically lasts for the first 2 to 3 weeks following LTx. However, it can rarely become a severe occurence, leading to graft loss of organ function and non function [17]. PRI is characterised by endothelial cell swelling (particularly affecting the sinusoids), central vein-based parenchymal injury (including hepatocyte ballooning and apoptosis, neutrophil aggregates, and parenchymal necrosis), and cholestatic features. Present study results showed that liver damage and preservation improved during cold and warm ischaemia durations. Other studies have found that up to 10% of transplanted livers may suffer from this condition [18].
ABO-incompatible transplants have been associated with AMR, an immune-mediated disease caused by donor sensitisation. Due to the general immunological resistance of the liver to the AMR mechanism of injury, hepatic AMR occurs in contrast to other transplanted organs (particularly the kidneys) [19]. According to the 2016 Banff Working Group on liver allograft pathology, AMR can be acute or chronic [4]. In this study, one case of acute AMR was found, with the timing of the biopsy taken 10 days post-transplant.
Improved surgical methods and immunosuppressive drugs have boosted long-term survival following LTx, leading to an increased recurrence rate of primary illnesses. One unique case (2.22%) involving a 38-year-old male with liver cirrhosis, Grade 2 esophageal varices, splenomegaly, and a family history of liver disease caused by persistent arsenic exposure. Regarding hepatoportal sclerosis, the hepatectomy specimen showed a vascular neoplasm with areas of cavernous haemangioma, epithelioid haemangioendothelioma, and diffusely infiltrating angiosarcoma. Post-Transplant Liver Biopsy (PTLB) revealed a recurrence of the vascular neoplasm eight months after the transplant.
Ethanol-induced hepatic injury was diagnosed in one case (2.22%) among allograft livers. There was no chronic rejection observed in this series of transplant recipients. Due to our patient’s shorter follow-up period, no cases of post-liver transplant cirrhosis have been documented.
Every critical step in liver transplantation involves pathologists, including defining liver graft function, identifying rejection, and recognising acute as well as long-term post-transplant complications.
Authors anticipate the approach of a novel era in liver histology evaluation, particularly in LTx. By combining multiplex immunohistochemistry with tissue-tethered digital analysis, next-generation pathology can extract precise single-cell morphometric, phenotypic, and spatial characteristics from biopsies [20]. This will generate new data types that are improved by their link with tissue architecture. In the future, novel pathology methodologies will reveal previously unknown information regarding transplant pathology.
Limitation(s)
This study has some limitations. First, it had a small sample size. Second, the cases were drawn from a single institution. Third, there was a potential for selection bias. Finally, there was a lack of generalisability to different populations.
Conclusion(s)
This study offers a comprehensive analysis of critical complications following LTx by shedding light on the types, frequency, and timing of these complications. This information is essential for guiding therapeutic interventions and ensuring the long-term viability of transplanted organs. Clinicians, pathologists, and transplant teams must collaborate closely to optimise patient outcomes, manage complications effectively, and enhance long-term graft survival. Future research in transplant pathology holds promise for further advancements.
CLD: Chronic liver disease; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus;
HBV: Hepatitis B virus
RAI: Rejection activity index; IHC: Intrahepatic cholestasis; ICP: Isolated central perivenulitis; HSV: Herpes simplex virus; DHF: Dengue haemorrhagic fever; PRI: Preservation reperfusion injury; AMR: Antibody-mediated rejection