Historically, tumours of the epidermal appendages have been classified into four groups that exhibit histologic features analogous to hair follicles, sebaceous glands, apocrine glands, and eccrine glands. In general, this type of diagnostic nosology, based on the microscopic attributes of appendageal structures, has been practical for the classification of adnexal tumours [1]. Histogenesis is a concept that implies that the histologic appearance of a tumour is similar to the histology of the organ or structure from which the tumour arose (the “cell of origin”). These concept of appendageal tumourigenesis is being modified to pertain to the mechanisms governing the proliferation and differentiation of pluripotent cells that may be situated within multiple compartments in the epidermis and associated adnexa. Generally, tumours are not derived directly from mature (differentiated, postmitotic) cells; rather, they originate from undifferentiated cells present within the epidermis or its appendageal structures [1]. In addition to benign tumours, there are carcinomas of the epidermal appendages, which have the potential to metastasise. Three types of glandular carcinoma are recognised: carcinoma of sebaceous glands, eccrine glands, and apocrine glands [1].
The vast majority of SAT differentiate only along one adnexal line, resulting in the formation of a reasonably distinct type whose structure, cytochemistry, and immunohistochemistry can be correlated with those of the corresponding adnexa [2]. However, since all cutaneous adnexa share the same origin, it is not surprising that the tumours arising from them exhibit many common features [2].
Skin adnexa are composed of various types of cells that can give rise to a wide spectrum of tumours. SATs are rare, and their morphological features, as well as their correlation with age, sex, site, and location, can be better understood through a cross-sectional study.
As no study on SATs has been conducted in a tertiary care centre in Chhattisgarh so far, the present study was undertaken to investigate the histopathological spectrum of SATs and to analyse the demographic profile in terms of age, sex, and anatomical location.
Materials and Methods
The present study was a cross-sectional study conducted over a period of five years, from August 2017 to July 2022, in the Department of Pathology at Pt. JNM Medical College, Raipur, C.G. Ethical considerations were approved by the ethics committee (Reference No. MC/ETHICS/2022/80, Date: 14/03/2022).
Inclusion criteria: All skin biopsies with a clinical diagnosis of adnexal tumours received in the Department of Pathology during the study period were included in the study.
Exclusion criteria: Inadequate, inconclusive, and autolysed skin biopsies were excluded from this study.
Study Procedure
All histologically diagnosed cases of SATs that came to the Department of Pathology during the study period were included. Relevant data, such as demographic profiles, anatomical locations, and clinical diagnosis, were recorded. Slides were examined microscopically for histomorphological features.
All cases were categorised as benign or malignant and classified according to differentiation as per the WHO Classification of Skin Tumours 2017 [7].
Statistical Analysis
The data was entered into Microsoft Excel, and results were presented in the form of frequency distributions in terms of numbers and percentages.
Results
The study included 51 cases across all age groups. Of the total, 22 were male and 29 were female, resulting in a male-to-female ratio of 1:1.3. Ages ranged from 13 to 90 years, with a mean age of 46.9 years. The maximum number of cases was observed in the age group of 41-60 years, which constituted 17 cases (33.3%) [Table/Fig-1].
Age wise distribution of skin adnexal tumours.
| Age group (years) | n (%) |
|---|
| 0-20 | 6 (11.8) |
| 21-40 | 16 (31.4) |
| 41-60 | 17 (33.3) |
| 61-80 | 11 (21.6) |
| 81-100 | 1 (2.0) |
Benign tumours accounted for 35 cases (68.63%), while malignant tumours constituted 16 cases (31.37%). Tumours measuring less than 2.5 cm made up the largest number of cases, totaling 25 (49.01%) [Table/Fig-2].
Distribution of skin adnexal tumour according to their size.
| Size of tumour (cm) | n (%) |
|---|
| <2.5 | 25 (49.01) |
| 2.5-5.0 | 19 (37.25) |
| >5.0 | 07 (13.74) |
Distribution of SAT is shown in [Table/Fig-3]. Among the 51 SAT, those with follicular differentiation were the most common, comprising 25 cases (49.02%), followed by sebaceous gland differentiation with 13 cases (25.49%), and sweat gland differentiation with 12 cases (23.53%). Paget’s disease of the nipple accounted for one case (1.96%) [Table/Fig-4].
Distribution of skin adnexal tumour according to their location.
Frequency distribution of individual skin adnexal tumours according to their differentiation.
| Differentiation (No. and % of cases) | Skin adnexal tumour | n (%) |
|---|
| Follicular differentiation n=25 (49.02%) | Pilomatrixoma | 13 (25.49) |
| Trichilemmal cyst | 02 (3.92) |
| Trichoepithelioma | 02 (3.92) |
| Trichilemmoma | 03 (5.88) |
| Trichilemmal carcinoma | 03 (5.88) |
| Trichoblastic carcinoma | 01 (1.96) |
| Pilomatrix carcinoma | 01 (1.96) |
| Sebaceous differentiation n=13 (25.49%) | Sebaceous adenoma | 04 (7.84) |
| Sebaceoma | 02 (3.92) |
| Sebaceous carcinoma | 07 (13.72) |
| Sweat gland differentiation n=12 (23.53%) | Hideradenoma | 07 (13.73) |
| Adenoid cystic carcinoma | 02 (3.92) |
| Poroma | 01 (1.96) |
| Syringocystadenoma papilliferum | 01 (1.96) |
| Hidradenocarcinoma | 01 (1.96) |
| Site specific tumour n=01 (1.96%) | Mammary Paget’s disease | 01 (1.96) |
| Total | 51 (100) |
Among all SATs, pilomatrixoma was the most common benign tumour, constituting 13 cases (25.49%), followed by hidradenoma with seven cases (13.73%), sebaceous adenoma with four cases (7.84%), and trichilemmoma with three cases (5.88%). Trichilemmal cyst, trichoepithelioma, and sebaceoma each accounted for two cases (3.92%), while poroma and syringocystadenoma papilliferum each constituted one case (1.96%) [Table/Fig-5].
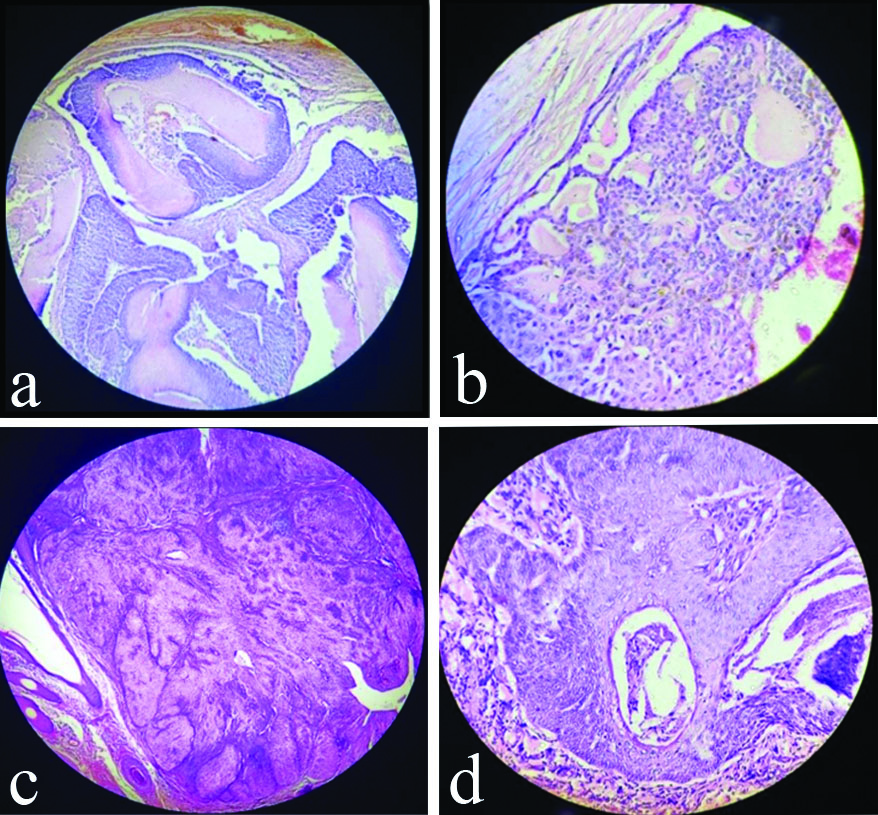
Histopathological pictures of benign SAT.
a) Pilomatrixoma-Tumour composed of epithelial island of basaloid cells in the centre with fibrous cellular stroma (H&E, 4x); b) Nodulocystic Hidradenoma-Tumour cells are round to oval to polyhedral with clear to basophilic cytoplasm with small dark nuclei with hyalinisation of stroma (H&E, 40x); c) Sebaceous Adenoma- A well circumscribed mature sebaceous lobules surrounded by fibrous capsule (H&E, 4x); d) Trichilemmoma- Bulbous tumour of basaloid cells with stromal clefting and peripheral palisading (H&E, 10x)
Among the malignant SAT, sebaceous carcinoma was the most common, comprising seven cases (13.72%), followed by trichilemmal carcinoma with four cases (7.84%), and adenoid cystic carcinoma with two cases (3.92%). Pilomatrix carcinoma, malignant nodular hidradenoma, and Paget’s disease of the nipple each accounted for one case (1.96%) [Table/Fig-6].
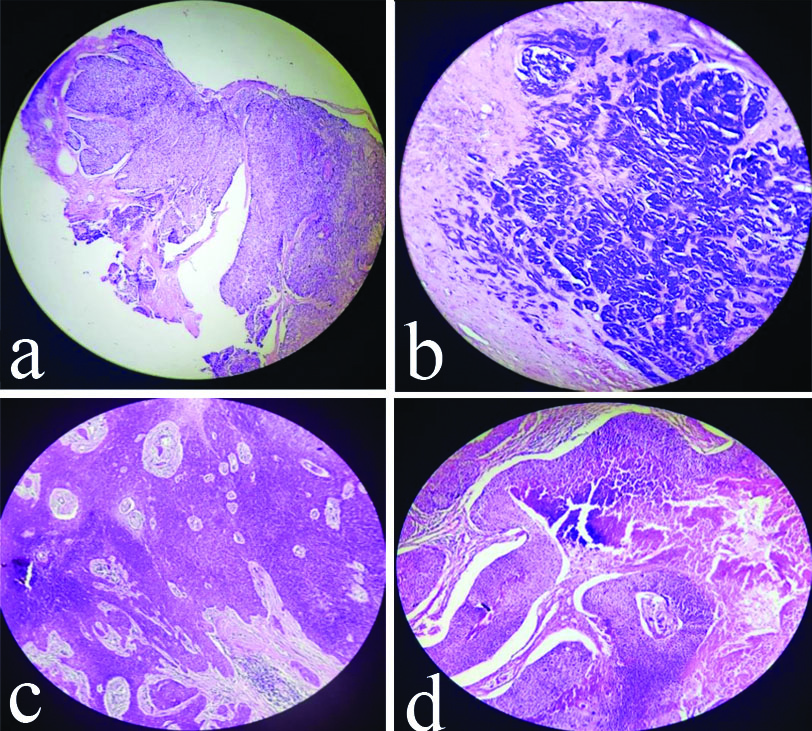
Histopathological pictures of malignant SAT.
a) Sebaceous Carcinoma- Dermal based lobulated tumour with focal epidermal connection. Tumour composed of sheets and lobules of basaloid cells separated by thin fibrovascular stroma (H&E, 4x); b) Adenoid Cystic Carcinoma-Tumour showing cribriform appearance (H&E, 10x); c) Trichilemmal Carcinoma- Composed of broad lobulated sheets of basaloid tumour cells with infiltrative growth pattern at the margin of tumour (H&E, 4x); d) Pilomatrix Carcinoma- Lobules of matrical cells with foci of necrosis and calcification and pilar type keratinisation seen (H&E, 4x)
Discussion
SATs represent a large and diverse group of benign and malignant tumours that exhibit morphological differentiation toward one of the various types of adnexal epithelium present in normal skin, including follicular differentiation, sebaceous differentiation, and sweat gland differentiation. A total of 51 cases were included in this study, comprising 22 males (43.14%) and 29 females (56.86%), resulting in a male-to-female ratio of 1:1.3, with a slight female predominance.
In a similar study by Pujani M et al., 25 cases were included, yielding a male-to-female ratio of 0.92:1 [8]. In the study by Sameer MA et al., which included 27 cases, there were 20 females and seven males, resulting in a male-to-female ratio of 1:2.8 [9]. Other studies and their male:female ratio is shown in [Table/Fig-7] [11].
Comparison of male to female ratio in various studies with present study [8-12].
| Study | M:F |
|---|
| Pujani M et al., [8] (2016), Delhi | 0.92 |
| Sameer MA et al., [9] (2017), Maharashtra | 1:2.8 |
| Agarwal S et al., [10], (2018), Madhya Pradesh | 1:1.4 |
| Thakuria KS et al., [11] (2020), Assam | 1:1.5 |
| Parvati M et al., [12] (2020), Andhra Pradesh | 1:1.4 |
| Present study, 2024 | 1: 1.3 |
In the present study, the age of the patients ranged from 13 to 90 years. Out of 51 cases, the majority were in the 41-to-60-year age group, accounting for 33.33%. The mean±SD age was observed to be 46.90±8 years. Pujani M et al., reported on 25 cases aged between 8 and 58 years, with the peak incidence occurring in the 21 to 40-year age group (56%), and a mean age of 32 years [8]. Valand AG et al., studied 90 cases, noting that the maximum age group was between 21 and 40 years, with a mean age of 35.8 years [13]. Amin J et al., examined 92 cases aged between 1 and 75 years, finding that the majority were in the 21 to 40-year age group [14]. Garima et al., observed 57 cases, with the maximum age group being 41 to 60 years (31.2%), and a mean age of 45 years [Table/Fig-8] [15].
Comparison of commonest age group in various studies with present study [8,13-15].
| Study | Commonest age group (years) |
|---|
| Pujani M et al., [8] (2016), Delhi | 21-40 |
| Valand AG et al., [13] (2016), Maharashtra | 21-40 |
| Amin J et al., [14] (2017), Maharashtra | 21-40 |
| Garima et al., [15] (2019), Rajasthan | 41-60 |
| Present study | 41-60 |
Among the 51 cases, there were 35 benign cases, comprising 68.63%, while 16 cases were malignant, representing 31.37%. A similar study by Sharma A et al., identified 56 cases, of which 45 (80.36%) were benign and 11 (19.64%) were malignant [16]. Pujani M et al., found that out of 25 cases, 24 (96%) were benign and 1 (4%) was malignant [8]. Agarwal S et al., studied 47 cases and observed that 42 (89.36%) were benign while 5 (10.63%) were malignant [10]. Garima et al., noted that of 57 cases, 55 (96.5%) were benign and 2 (3.5%) were malignant [15]. Omar AM and Osman NA reported on 18 cases, noting that 17 (94.4%) were benign and 1 (5.6%) was malignant [Table/Fig-9] [6].
Comparison of frequency of benign and malignant adnexal tumour in various studies with present study [6,8,10,15,16].
| Study | Benign tumour (%) | Malignant tumour (%) |
|---|
| Sharma A et al., [16] (2014), Maharashtra | 80.36 | 19.64 |
| Pujani M et al., [8] (2016), Delhi | 96 | 4 |
| Agarwal S et al., [10] (2018), Madhya Pradesh | 89.36 | 10.64 |
| Garima et al., [15] (2019), Rajasthan | 96.5 | 3.5 |
| Omar AM and Osman NA [6] (2020), Egypt | 94.4 | 5.6 |
| Present study, 2024 | 68.63 | 31.37 |
Of the 51 SAT, the most common were those of follicular differentiation, constituting 25 cases (49.02%), followed by sebaceous gland differentiation with 13 cases (25.49%), and sweat gland differentiation, which included 12 cases (23.53%). Additionally, there was 1 case (1.96%) of a site-specific tumour, namely Mammary Paget’s disease. In a similar study, Suri J et al., observed 66 cases, of which 25 (37.87%) were tumours of follicular differentiation, 19 (28.78%) were of sebaceous differentiation, and 14 (21.21%) were of sweat gland differentiation [17]. Valand AG et al., reviewed 90 cases, reporting that 43 (47.78%) were hair follicle tumours, 23 (25.56%) were sebaceous gland tumours, and 24 (26.66%) were sweat gland tumours [Table/Fig-10] [13].
Comparison of line of differentiation of adnexal tumour in various studies with present study [13,17].
| Study | Follicular | Sweat gland | Sebaceous |
|---|
| Suri J et al., [17] (2016), Jammu | 37.87% | 21.21% | 28.78% |
| Valand AG et al., [13] (2016), Maharashtra | 47.78% | 26.66% | 25.56% |
| Present study, 2024 | 49.02% | 23.53% | 25.49% |
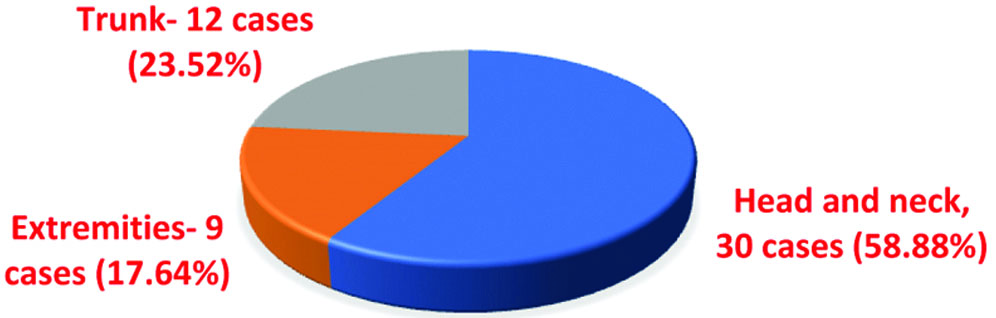
In the present study, the head and neck region was the most common location of tumours, comprising 30 cases (58.82%)-19 in the face, 9 on the scalp, and 2 in the neck. The trunk region was the second most common site, accounting for 12 cases (23.53%), followed by the extremities, which constituted 9 cases (17.65%).
Comparison of present sty with other studies are shown in [Table/Fig-11] [6,8,9,13,14].
Comparison of adnexal tumour at different site in various studies with present study [6,8,10,14,15].
| Study | Head and neck | Extremities | Trunk |
|---|
| Pujani M et al., [8] (2016), Delhi | 72% | 20% | 10% |
| Amin J et al., [14] (2017), Srinagar | 60.9% | 18.45% | 20.6% |
| Agarwal S et al., [10] (2018), Madhya Pradesh | 57.44% | 17.02% | 17.02% |
| Garima et al., [15] (2019), Rajasthan | 47.5% | 38.3% | -- |
| Omar AM and Osman NA [6] (2020), Egypt | 44.4% | 33.3% | 22.3% |
| Present study, 2024 | 58.82% | 17.65% | 23.53% |
In the present study, tumours smaller than 2.5 cm constituted 25 cases (49.01%), followed by tumours measuring between 2.5 cm and 5.0 cm, which accounted for 19 cases (37.25%). Tumours larger than 5.0 cm comprised seven cases (13.74%). A similar study by Pujani M et al., observed 25 cases, of which 13 (52%) tumours were smaller than 1.5 cm, 7 (28%) were between 1.6 cm and 2.5 cm, 4 (16%) were between 2.6 cm and 4.0 cm, and a single case (4%) was larger than 4 cm [8].
In the present study, among tumours with follicular differentiation, pilomatrixoma was the most common benign adnexal tumour, comprising 13 cases (25.49%). This was followed by trichilemmoma and trichilemmal carcinoma, each comprising three cases (5.88%). prichoepithelioma and trichilemmal cyst each accounted for two cases (3.92%), while trichoblastic carcinoma and pilomatrix carcinoma each comprised one case (1.96%). A similar study conducted by Pujani M et al., found that out of 25 cases, 4 (16%) were pilomatrixoma, 2 (8%) were trichilemmal cyst, and there was a single case (4%) of trichoepithelioma [8]. Suri J et al., recorded 66 cases, among which pilomatrixoma was the most common follicular tumour, with 13 cases (19.69%). This was followed by seven cases of trichilemmal cyst, three cases of trichoepithelioma, and a single case each of trichilemmoma and trichofolliculoma [17]. Valand AG et al., studied 90 cases and found that both pilomatrixoma and trichoepithelioma were the most common tumours, each comprising 18 cases [Table/Fig-12] [13].
Comparison of tumour of follicular differentiation in various studies with present study [8,13,17].
| Name of the lesion | Studies showing percentage distributions (%) |
|---|
| Present study, 2024 | Pujani M et al., [8] (2016), Delhi | Suri J et al., [17] (2016), Jammu | Valand AG et al., [13] (2016), Maharashtra |
|---|
| Pilomatrixoma | 25.49 | 16 | 19.69 | 20 |
| Trichelemal cyst | 3.92 | 8 | 10.6 | |
| Trichoepithelioma | 3.92 | 4 | 4.5 | 20 |
| Trichelemoma | 5.88 | | 1.5 | .... |
| Trichoblastic carcinoma | 1.96 | .... | .... | .... |
| Trichelemal carcinoma | 5.88 | .... | .... | .... |
| Pilomatrix carcinoma | 1.96 | .... | .... | .... |
In terms of sebaceous differentiation, sebaceous carcinoma was the most common malignant tumour, comprising seven cases (13.73%) of the total SATs. This was followed by sebaceous adenoma with four cases (7.84%) and sebaceoma with two cases (3.92%). A similar study by Pujani M et al., found that out of 25 cases, sebaceous carcinoma was the only malignant tumour encountered [8]. Valand AG et al., and Suri J et al., also observed that sebaceous carcinoma was the most common malignant tumour, comprising 2.66% and 5.56% of their respective studies [13,17]. Amin J et al., recorded 92 cases, among which sebaceous carcinoma was the most common malignant tumour, accounting for three cases (3.3%), followed by sebaceous adenoma with two cases (2.17%) [Table/Fig-13] [14].
Comparison of tumour of sebaceous gland differentiation in various study with present study [8,13,14,17].
| Study | Sebaceous adenoma | Sebaceoma | Sebaceous carcinoma |
|---|
| Pujani M et al., [8] (2016), Delhi | -- | -- | 4% |
| Suri J et al., [17] (2016), Jammu | -- | -- | 2.66% |
| Valand AG et al., [13] (2016), Maharashtra | -- | -- | 5.56% |
| Amin J et al., [14] (2017), Srinagar | 2.17% | -- | 3.26% |
| Present study, 2024 | 7.84% | 3.92% | 13.73% |
Regarding sweat gland differentiation, hidradenoma was the most common tumour, comprising seven cases (13.73%) of total sweat gland differentiation. This was followed by adenoid cystic carcinoma with two cases (3.92%), and poroma, syringocystadenoma papilliferum, and hidradenocarcinoma, each comprising one case (1.96%). A similar study conducted by Pujani M et al., found that out of 25 cases, hidradenoma papilliferum was the most common sweat gland tumour, comprising four cases (16%), followed by hidradenoma and syringocystadenoma papilliferum, each comprising one case (4%) [8]. Suri J et al., recorded two cases each of poroma and syringocystadenoma papilliferum (4.54%) [Table/Fig-14] [17].
Comparison of tumour of sweat gland differentiation in various study with present study [8,17].
| Study | Hidradenoma | Adenoid cystic carcinoma | Poroma | Syringocystadenoma papiliferum | Malignant nodular hidradenoma |
|---|
| Pujani M et al., [8] (2016), Delhi | 4% | -- | -- | 4% | -- |
| Suri J et al., [17] (2016), Jammu | -- | -- | 4.54% | 4.54% | -- |
| Present study, 2024 | 13.73% | 3.92% | 1.96% | 1.96% | 1.96% |
Limitation(s)
There was a lack of clinical correlation, and the patient’s follow-up could not be conducted. Although SATs are uncommon tumours, the small sample size also limited the study. Research with a larger sample size could provide more insights into SAT.
Conclusion(s)
SATs present a diagnostic challenge for both clinicians and pathologists due to their diverse clinical presentations and overlapping histopathological features. Present study emphasises that the histomorphological features of adnexal tumours and histopathological examination remain the gold standard for establishing a diagnosis, despite the expanding use of and reliance on special ancillary techniques such as Immunohistochemistry (IHC). In a few cases, IHC plays an important role in diagnosis. The majority of tumours can be classified into different subgroups based on light microscopy.