The neonates are very sensitive and vulnerable individuals. Prematurity and prolonged Neonatal Intensive Care Unit (NICU) stays lead to the use of multiple medications, total parenteral nutrition, and various intravenous infusions. NICU patients are prescribed a median range of 3 to 11 medications, with some babies requiring as many as 40 [1]. The World Health Organisation (WHO) recognises pharmacists as essential resources for the safe and effective use of medicines [2]. Pharmacist-led interventions can improve medication management in the NICU and have been shown to reduce medication errors [3].
A classification scheme for Drug-related Problems (DRPs) was developed at the Pharmaceutical Care Network Europe (PCNE) conference in January 1999 and is regularly validated and adapted [4]. The current version is V9.1 (February 2020). This scheme helps healthcare professionals document DRP information in the pharmaceutical care process. A DRP is defined as “an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes” [4].
The DRPs are prevalent in NICUs, with the majority arising from suboptimal treatment, primarily due to incorrect dose selection [5]. There is very little written about DRPs in the NICU. Authors hereby, conducted a study to assess the status of DRPs in a tertiary care teaching hospital where residents are assigned to the NICU. The present study is necessary to identify common DRPs in institutional settings and improve the quality of care. It can also draw the attention of authorities to the need for a clinical pharmacologist to be part of the NICU team or for a team member to be specifically designated for this work. The aims and objectives of present study were to provide a comprehensive approach to understanding and mitigating DRPs in neonates in the NICU, ultimately enhancing patient safety and care quality.
Materials and Methods
This prospective observational study was conducted from June 2023 to August 2023 in a tertiary-level NICU at JNU Hospital, Jaipur, Rajasthan, India. The study received approval from the Hospital Ethics Committee (IEC approval letter No. JNUIMSRC/IEC/202/87).
Inclusion criteria: All neonates admitted to the NICU during the study period were enrolled.
Exclusion criteria: Neonates admitted for less than 24 hours and those who had not been prescribed any medications were excluded.
Study Procedure
Data collection: Baseline characteristics such as age, sex, birth weight, and gestational age of all enrolled newborns were recorded. A clinical pharmacology doctor assigned to the NICU reviewed the medication charts and assessed them using the Micromedex Neofax Essentials 2020 (USA-based neonatal formulary) [6]. He documented all DRPs using the (PCNE V9.1) [4]. Permission was obtained to use (PCNE V9.1).
The (PCNE V9.1) has five main classifications: problems, causes of DRP, planned interventions, level of acceptance, and status of the DRP (outcome of intervention). Each classification is further divided into domains and sub-domains.
The problem is categorised into three primary domains, which are further divided into six sub-domains. The causes of the DRP are branched into nine domains, which are then subdivided into 38 sub-domains. Similarly, the Planned Intervention has five primary domains and 17 sub-domains. The acceptance of intervention proposals consists of three domains and 10 sub-domains. The status of the DRP (Outcome of Intervention) is classified into four domains and four sub-domains [4].
The types of problems were identified, and a list of DRPs and their causes was compiled. The clinical pharmacology intern reported the DRPs, their causes, and the planned interventions to the treating physician according (PCNE V9.1). A list of planned interventions was also created. There could be multiple causes of DRPs and planned interventions for a single DRP. The intern documented the acceptance of the intervention proposals and the final status of the DRPs, which reflects the outcome of the intervention.
Statistical Analysis
The presentation of categorical variables was done in the form of numbers and percentages (%). On the other hand, quantitative data with a normal distribution were presented as mean±SD, while data with a non normal distribution were presented as medians with the 25th and 75th percentiles (interquartile range). The normality of the data was checked using the Kolmogorov-Smirnov test. In cases where the data was not normally distributed, non parametric tests were applied. The following statistical tests were used for the results: the Spearman rank correlation coefficient was employed to assess the correlation between the length of hospital stay (in days) and the number of DRPs. The incidence of DRPs was compared using the 95% Confidence Interval (CI) of the incidence rate. Data entry was performed using Microsoft Excel, and the final analysis was conducted with the Statistical Package for Social Sciences (SPSS) software, IBM Corporation, Chicago, USA, version 25.0. A p-value of <0.05 was considered statistically significant for all analysis.
Results
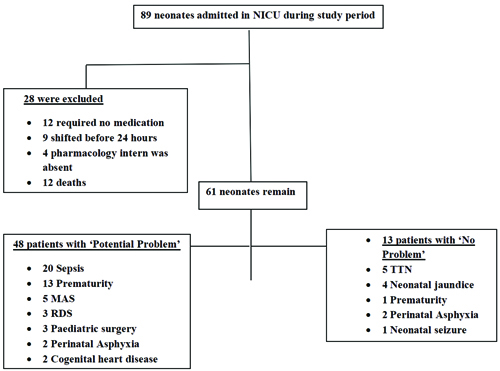
A total of 61 newborns were enrolled out of 89 admissions. Nine were transferred before completing 24 hours of stay in the NICU, and 12 did not require any medications. Four were missed because the clinical pharmacology intern was on leave, and three died.
Potential problems were identified in 48 patients (78.69%), while no problems were observed in 13 patients (21.31%). The neonates with potential problems included those with sepsis 20 (32.78%), prematurity 13 (21.31%), meconium aspiration syndrome 5 (8.19%), respiratory distress syndrome 3 (4.91%), paediatric surgery 3 (4.91%), perinatal asphyxia 2 (3.27%), and congenital heart diseases 2 (3.27%). The neonates identified with no problems had conditions such as Transient Tachyapnoea in the Newborns (TTN) 5 (8.19%), neonatal jaundice 4 (6.55%), perinatal asphyxia 2 (3.27%), prematurity 1 (1.63%), and neonatal seizures 1 (1.63%) [Table/Fig-1].
In the present study, males comprised 34 (55.74%) of the participants, outnumbering females. The mean gestational age was 35.2±3.2 weeks, and the mean birth weight was 2257.87±665.78 grams. The mean length of NICU stay was 8.95±7.99 days, ranging from 1 to 50 days. The mean number of DRPs per patient was 1.57±1.42. The correlation coefficient between the length of hospital stay and the number of DRPs was found to be 0.472, which was statistically significant (p-value of 0.0001) [Table/Fig-2].
Baseline characteristics of study participants.
| Variables | Values |
|---|
| Gender |
| (Male), n (%) | 34 (55.74%) |
| Female | 27 (44.26%) |
| Age (days), Mean±SD | 3.11±4 |
| Gestational age (Weeks), Mean±SD | 35.2±3.2 |
| ≥32 weeks | 53 (86.89%) |
| Birth weight (grams), Mean±SD | 2257.87±665.78 |
| Length of hospital stay (days), Mean±SD, Median (25th-75th percentile), Range (days) | 8.95±7.99, 6 (4-10), 1-50 |
| Death, n (%) | 3 (4.92%) |
| Patients with DRPs, n (%) | 47 (77.05%) |
| Total number of DRPs, n | 98 |
| Incidence of DRP (95% CI) | 1.57 (1.27 to 1.92) |
| DRP per patient, Mean±SD | 1.57±1.42 |
| Correlation coefficient of length of hospital stay and number of DRPs (p-value) | 0.472 (0.0001) |
DRPs were observed in 47 (77.05%) patients. The total number of DRPs was 96, with their distribution as follows: 19 (31.15%) patients had only one DRP, while 1 (1.64%) patient had a maximum of seven DRPs. The remaining patients fell in between these minimum and maximum values; 17 (27.87%) patients had two DRPs, 6 (9.84%) patients had three DRPs, 2 (3.28%) patients had 4 DRPs, and 2 (3.28%) patients had five DRPs [Table/Fig-3].
| No. of patients | Frequency of DRP(per patient) | Total DRP |
|---|
| DRP present | 47 (77.05%) | | |
| 19 (31.15%) | 1 | 19 |
| 17 (27.87%) | 2 | 34 |
| 6 (9.84%) | 3 | 18 |
| 2 (3.28%) | 4 | 8 |
| 2 (3.28%) | 5 | 10 |
| 1 (1.64%) | 7 | 7 |
| DRP absent | 14 (22.95%) | 0 | |
| Total | 61 (100%) | | 96 |
The most common domain of problems was ‘others,’ which accounted for 68 (69.39%) DRPs. The most prevalent sub-domain was ‘unnecessary drug treatment’ in 63 (64.29%) DRPs. The ‘treatment effectiveness’ domain was seen in 30 (30.61%) DRPs, with the most common sub-domain being ‘effect of drug treatment not optimal’ in 28 (28.57%) DRPs.
The most common cause of DRPs was ‘dose selection,’ which was responsible for 105 (62.87%) DRPs. The most frequent sub-domain within this category was ‘drug dose of a single active ingredient too high’ in 40 (23.95%) DRPs, followed by ‘drug dose too low’ in 30 (17.96%) DRPs. The second most common cause of DRPs was ‘drug use process,’ which accounted for 61 (36.54%) DRPs, with the most prevalent sub-domain being ‘drug over-administered by a health professional’ in 40 (23.95%) DRPs. The next common sub-domain was ‘drug under-administered by a health professional’ in 15 (8.98%) DRPs.
The most common planned intervention was at the prescriber level, which was noted in 99 (99%) DRPs, with the most frequent sub-domain being ‘Intervention discussed with prescriber’ in 90 (91.81%) DRPs.
The acceptance of the intervention proposals included ‘Intervention accepted’ in 96 (97.96%) of DRPs, while ‘Intervention not accepted’ occurred in 2 (2.05%) DRPs. Among the interventions not accepted, one (1.02%) was due to ‘not feasible’ and another (1.02%) due to ‘no agreement.’
The outcome of the intervention was ‘problem totally solved’ in 69 (70.41%) DRPs, ‘problem partially solved’ in 10 (10.20%) DRPs, ‘problem not solved’ in 17 (17.35%) DRPs, and ‘problem status unknown’ in 2 (2.04%) DRPs. The ‘problem not solved’ cases were due to ‘lack of cooperation from the prescriber’ in 9 (9.18%) DRPs and ‘no need or possibility to solve the problem’ in 6 (6.12%) DRPs [Table/Fig-4].
Distributions of various parameters of PCNE classification of DRPs.
| I | Problem | |
|---|
| P1 | Treatment effectiveness | 30 (30.61%) |
| P1.1 | No effect of drug treatment despite correct use | 0 |
| P1.2 | The effect of drug treatment not optimal | 28 (28.57%) |
| P1.3 | Untreated symptoms or indication | 2 (2.04%) |
| P2 | Treatment safety | 0 |
| P2.1 | Adverse drug events (possibly) occurring | 0 |
| P3 | Others | 68 (69.39%) |
| P3.1 | Unnecessary drug-treatment | 63 (64.29%) |
| P3.2 | Unclear problem/complaint | 5 (5.10%) |
| II | Cause of DRP | |
| C1 | Drug selection | 0 |
| C1.1 | Inappropriate drug according to guidelines/formulary | 0 |
| C1.2 | No indication for drug | 0 |
| C1.3 | Inappropriate combination of drugs, or drugs and herbal medications, or drugs and dietary supplements | 0 |
| C1.4 | Inappropriate duplication of therapeutic group or active ingredient | 0 |
| C1.5 | No or incomplete drug treatment inspite of existing indication | 0 |
| C1.6 | Too many different drugs/active ingredients prescribed for indication | 0 |
| C2 | Drug form | 1 (0.59%) |
| C2.1 | Inappropriate drug form/formulation (for this patient) | 1 (1.02%) |
| C3 | Dose selection | 105 (62.87%) |
| C3.1 | Drug dose too low | 30 (17.96%) |
| C3.2 | Drug dose of a single active ingredient too high | 40 (23.953%) |
| C3.3 | Dosage regimen not frequent enough | 12 (7.18%) |
| C3.4 | Dosage regimen too frequent | 21 (12.57%) |
| C3.5 | Dose timing instructions wrong, unclear or missing | 2 (1.19%) |
| C4 | Treatment duration | 0 |
| C4.1 | Duration of treatment too short | 0 |
| C4.2 | Duration of treatment too long | 0 |
| C5 | Dispensing | 0 |
| C5.1 | Prescribed drug not available | 0 |
| C5.2 | Necessary information not provided or incorrect advice provided | 0 |
| C5.3 | Wrong drug, strength or dosage advised | 0 |
| C5.4 | Wrong drug or strength dispensed | 0 |
| C6 | Drug use process | 61 (36.54%) |
| C6.1 | Inappropriate timing of administration or dosing intervals by a health professional | 5 (2.99%) |
| C6.2 | Drug under-administered by a health professional | 15 (8.98%) |
| C6.3 | Drug over-administered by a health professional | 40 (23.95%) |
| C6.4 | Drug not administered at all by a health professional | 0 |
| C6.5 | Wrong drug administered by a health professional | 0 |
| C6.6 | Drug administered via wrong route by a health professional | 1 (0.59%) |
| C7 | Patient-related | 0 |
| C7.1 | Patient intentionally uses/takes less drug than prescribed or does not take the drug at all for whatever reason | 0 |
| C7.2 | Patient uses/takes more drug than prescribed | 0 |
| C7.3 | Patient abuses drug (unregulated overuse) | 0 |
| C7.4 | Patient decides to use unnecessary drug | 0 |
| C7.5 | Patient takes food that interacts | 0 |
| C7.6 | Patient stores drug inappropriately | 0 |
| C7.7 | Inappropriate timing or dosing intervals | 0 |
| C7.8 | Patient unintentionally administers/uses the drug in a wrong way | 0 |
| C7.9 | Patient physically unable to use drug/formas directed | 0 |
| C7.10 | Patient unable to understand instructions properly | 0 |
| C8 | Patient transfer related | 0 |
| C8.1 | Medication reconciliation problem | 0 |
| C9 | Other | 0 |
| C9.1 | No or inappropriate outcome monitoring(incl. TDM) | 0 |
| C9.2 | Other cause; specify | 0 |
| No obvious cause | 0 |
| III | Planned intervention | |
| I0 | No intervention | 0 |
| I1 | At prescriber level | 99 (99%) |
| I1.1 | Prescriber informed only | 4 (4.08%) |
| I1.2 | Prescriber asked for information | 1 (1.02%) |
| I1.3 | Intervention proposed to prescriber | 4 (4.08%) |
| I1.4 | Intervention discussed with prescriber | 90 (91.84%) |
| I2 | At patient level | 0 |
| I2.1 | Patient (drug) counselling | 0 |
| I2.2 | Written information provided | 0 |
| I2.3 | Patient referred to prescriber | 0 |
| I2.4 | Spoken to family member/caregiver | 0 |
| I3 | At drug level | 1 (1%) |
| I3.1 | Drug changed to..... | 0 |
| I3.2 | Dosage changed to ..... | 0 |
| I3.3 | Formulation change to ..... | 0 |
| I3.4 | Instructions for use changed to ..... | 0 |
| I3.5 | Drug stopped or paused | 1 (1.02%) |
| I3.6 | Drug started | 0 |
| I4 | Another intervention or activity | 0 |
| I4.1 | Other intervention (specify) ..... | 0 |
| I4.2 | Side effect reported to authorities | 0 |
| IV | Acceptance of the intervention proposals | |
| A1 | Intervention accepted | 96 (97.96%) |
| A1.1 | Intervention accepted and fully implemented | 78 (79.59%) |
| A1.2 | Intervention accepted, partially implemented | 7 (7.14%) |
| A1.3 | Intervention accepted but not implemented | 7 (7.14%) |
| A1.4 | Intervention accepted, implementation unknown | 4 (4.08%) |
| A2 | Intervention not accepted | 2 (2.05%) |
| A2.1 | Intervention not accepted: not feasible | 1 (1.02%) |
| A2.2 | Intervention not accepted: no agreement | 1 (1.02%) |
| A2.3 | Intervention not accepted: other reason (specify) ..... | 0 |
| A3 | Other | 0 |
| A3.1 | Intervention proposed, acceptance unknown | 0 |
| A3.2 | Intervention not proposed | 0 |
| V | Status of the DRP (outcome of intervention) | |
| O0 | Problem status unknown | 2 (2.04%) |
| O1 | Problem totally solved | 69 (70.41%) |
| O2 | Problem partially solved | 10 (10.20%) |
| O3 | Problem not solved | 17 (17.35%) |
| O3.1 | Lack of cooperation of patients | 1 (1.02%) |
| O3.2 | Lack of cooperation of prescriber | 9 (9.18%) |
| O3.3 | Intervention not effective | 1 (1.02%) |
| O3.4 | No need or possibility to solve problem | 6 (6.12%) |
Discussion
The study was conducted to assess DRPs in the NICU. There have not been many studies conducted regarding DRPs in the NICU, and this research gap needs to be addressed. The present study assessed DRPs using the (PCNE V9.1) classification system, with the aim of increasing focus on DRPs and ultimately improving the quality of care.
In the present study, authors enrolled 61 newborns, of which 47 (77.05%) had DRPs. The total number of DRPs identified was 98. Leopoldino RD et al., conducted a similar study in a teaching hospital in Brazil using PCNE V6.2. Their study was larger than present, enrolling 600 neonates, of whom 359 (59.8%) had DRPs [5]. The total number of DRPs in their study was 1,142. Authors found that the most common problem was ‘unnecessary drug treatment,’ observed in 64.29% of DRPs, while they identified ‘effect of drug treatment not optimal’ in 52.8% of DRPs. The present study found ‘effect of drug treatment not optimal’ as the second most common problem, present in 28.57% of DRPs.
Additionally, the present study found that the most common cause of DRPs was ‘dose selection,’ accounting for 62.87% of DRPs. Similarly, Leopoldino RD et al., also found ‘inappropriate dose selection’ to be the most common cause of DRPs, present in 39.75% of cases [5]. Awoke M et al., conducted a similar study on neonates admitted with neonatal sepsis in a tertiary teaching hospital’s NICU in southwestern Ethiopia, using Cipolle’s method to classify DRPs [8]. They enrolled 201 neonates, of which 98 (48.8%) had DRPs, with a total of 121 DRPs identified. They found that the most common cause of DRPs was ‘dose is too high,’ present in 34.7% of DRPs, which is similar to the present findings. The next most common cause they identified was ‘need for additional drug therapy’ in 33.5% of DRPs, which is not part of the present classification. They also found ‘dose is too low’ in 19.8% of DRPs, while we identified it in 17.96% of DRPs [8].
Nascimento ARFD et al., conducted a study on DRPs in cardiac neonates under intensive care. They performed a cross-sectional study at a teaching maternity hospital in Brazil, enrolling a total of 122 neonates, of which 76.4% experienced DRPs. The total number of identified DRPs was 390. They found that the most common problem was ‘treatment effectiveness,’ which occurred in 49% of DRPs, followed by ‘adverse reactions’ in 46.7% of DRPs. In the present study, authors also identified ‘treatment effectiveness’ as a problem in 30.61% of cases; however, we did not find ‘adverse reactions’ to be a problem in any cases. Nascimento ARFD et al., reported that the most common cause of DRPs was the ‘drug use process’ in 32.6% of cases, while ‘dose selection’ was noted in 30.8% of DRPs [9].
Ahmed NA et al., conducted a study on pharmaceutical interventions for DRPs in the NICU, focusing on incidence, types, and acceptability. They included 316 neonates and identified a total of 1,723 DRPs, which occurred in 283 (89.6%) of the neonates. They also found ‘treatment effectiveness’ to be the most common problem (46.4%), while ‘dose selection’ was the most common cause, occurring in 61.9% of cases. This is similar to the present findings, where ‘treatment effectiveness’ was noted in 30.61% of cases and ‘dose selection’ in 62.87% [10].
The possible reason for the ‘suboptimal effect of drug treatment’ may be the incorrect selection of doses. One reason for incorrect dosing could be the physiology of newborns and their daily weight changes. Neonates have low plasma protein concentrations, a higher percentage of body water, and decreased liver metabolism and renal clearance [11]. Consequently, the risk of drug ineffectiveness or toxicity is always present in neonates [12,13].
Authors also identified a problem of ‘unnecessary drug treatment,’ which may arise from the involvement of multiple individuals in the treatment process, particularly in teaching hospitals where only resident doctors are present at times. Additionally, we found that drugs were sometimes over- or underadministered by healthcare professionals. This is a significant concern, as nursing staff may be rushed due to heavy workloads or shift changes, leading to inefficiencies or inexperience.
The planned intervention involved discussing the issues with the prescriber in 91.8% of the DRPs, and this was ‘accepted’ in 97.96% of cases. This led to a ‘total resolution’ of the problem in 70.41% of DRPs, indicating the need for a clinical pharmacology expert in a NICU. In other cases, the problem was ‘partially solved’ (10.20%) or ‘not solved’ (17.35%) due to a ‘lack of cooperation from the prescriber’ (9.18%), often stemming from their personal clinical experience or established regimens involving the same medications.
The present study found a positive correlation between the length of NICU stay and the number of DRPs, which was statistically significant (p-value of 0.0001). Previous studies by Ahmed NA et al., and Leopoldino RD et al., also found that DRPs were associated with an increased length of stay [10,14]. Adhering to strict guidelines and evidence-based practices in neonatology for NICU stays, as well as carefully managing medication initiation and dosing, may help mitigate these issues.
There has not been much work done on DRPs in neonatology, and we are among the first in India to address this issue. We have utilised the latest version of the PCNE Classification (V9.1), which provides a comprehensive and standardised framework for classifying and analysing DRPs. This version is more refined than those used in other studies conducted outside India, which relied on older versions of the PCNE or different methods.
The present study aims to improve the quality of care by identifying DRPs and their causes so that we can implement improvements. DRPs are prevalent in many NICUs. To address this, authors have initiated daily dose corrections, and consultants now cross-check the doses and dilutions written by residents. Additionally, a pharmacy intern is regularly assigned to the NICU to monitor drug doses noted by doctors, ensure their proper administration by nursing staff, and check for potential drug interactions.
Limitation(s)
The limitations of the present study include its small size and the fact that it was conducted at a single center. Furthermore, only one clinical pharmacologist was involved in the study, although he consulted with the NICU specialists for each DRP. Consequently, in his absence, authors may have overlooked some DRPs.
Conclusion(s)
The present study concludes that unnecessary and ineffective treatment is the most common DRP, while inappropriate dose selection is the most common cause of DRPs. More research on DRPs is needed in the future. Drug-related problems in neonates are a serious concern. Simultaneously, we need to address the length of stay in the NICU, as neonates who stay longer tend to be more seriously ill and require more cautious dosing of medications. The planned interventions were accepted by the treating physicians, and the problems were resolved in the majority of cases. This underscores the need for a clinical pharmacologist in NICUs.