Case Report
A 31-year-old male, employed as a clerk, presented with complaints of high-grade fever accompanied by chills for one month, along with an evening rise in temperature and fatigue. The patient also reported experiencing abdominal pain in the right hypochondriac region for the last 15 days, which has been increasing in intensity. He did not have complaints of vomiting, loose stools, cough, or bleeding manifestations such as petechial rash, haemoptysis, hematemesis, or melena. He mentioned a weight loss of 6 kg over the course of one month. His past medical history was unremarkable, and there was no significant family history or recent contact with TB. On general examination, the patient was pale and febrile, with a temperature of 101°F and tachycardia, with a pulse rate of 110/min. Upon abdominal examination, tenderness was noted in the right hypochondriac region, with a palpable liver and spleen, while other systemic examinations were within normal limits. Laboratory investigations revealed pancytopenia with raised inflammatory markers [Table/Fig-1].
Laboratory investigations at presentation on the first day of admission.
| Investigation | Value | Normal values |
|---|
| Hemoglobin (gm%) | 9.1 | 13.5-17.5 |
| Total leukocyte count (cells/mm3) | 3800(80% neutrophils) | 4000-11000 |
| Platelets (/mm3) | 1,09,000 | 150000-450000 |
| Reticulocyte count (%) | 1.6 | 0.5-1.5 |
| Reticulocyte index | 0.74 | 0.5-2.5 |
| Total bilirubin (mg/dL) | 0.76 | 0.1-1.2 |
| Direct bilirubin (mg/dL) | 0.44 | 0-0.3 |
| Aspartate transaminase (U/L) | 34 | 10-40 |
| Alanine transaminase (U/L) | 39 | 7-56 |
| Alkaline phosphatase (U/L) | 99 | 44-147 |
| Urea (mg/dL) | 22 | 10-50 |
| Creatinine (mg/dL) | 0.82 | 0.6-1.2 |
| Serum Sodium (mEq/L) | 136 | 135-145 |
| Serum Potassium (mEq/L) | 3.6 | 3.5-5.0 |
| C-reactive protein | 143 | >100 mg/L (high-risk) |
| Serum triglycerides (mg/dL) | 130 | Less than 150 |
| Erythrocyte sedimentation rate (mm/hr) | 79 | 0-22 |
| Ferritin (ng/dL) | 1376.12 | 30-400 |
| TIBC (μg/dL) | 201 | 240-450 |
| Iron (μg/dL) | 20 | 60-160 |
| Transferrin saturation (%) | 3.98 | 20-50 |
| Vitamin B12 (pg/mL) | 1823 | 180-914 |
| HIV/HbSAg/anti HCV | Non reactive | Non reactive |
| Dengue profile | Negative | Negative |
| Rapid malaria test | Negative | Negative |
| VDRL | Non reactive | Non reactive |
| Blood and urine C/S | No growth | No growth |
| Chikungunya | Negative | Negative |
| Weil Felix | Negative | Negative |
| Brucella | Negative | Negative |
| Leptospira | Negative | Negative |
TIBC: Total iron binding capacity; HIV: Human immunodeficiency virus; HbsAg; Hepatitis B surface antigen; HCV: Hepatitis C virus; C/S: Culture and sensitivity
A peripheral blood smear showed normal morphology of cells. Electrocardiography and chest radiography were normal. High-Resolution Computed Tomography (HRCT) of the chest showed no obvious abnormalities. Ultrasonography of the abdomen and pelvis revealed hepatomegaly (20 cm) with normal echotexture and splenomegaly (16 cm) with normal echotexture.
The patient was provisionally diagnosed with Pyrexia of Unknown Origin (PUO) accompanied by hepatosplenomegaly and pancytopenia, under evaluation, with a differential diagnosis of extrapulmonary TB, leptospirosis, and malaria. Initially, the patient was empirically started on tab doxycycline 100 mg BD for 10 days and given a course of injection artesunate 2.4 mg/kg for three days. However, the patient showed no improvement in symptoms and continued to experience fever spikes.
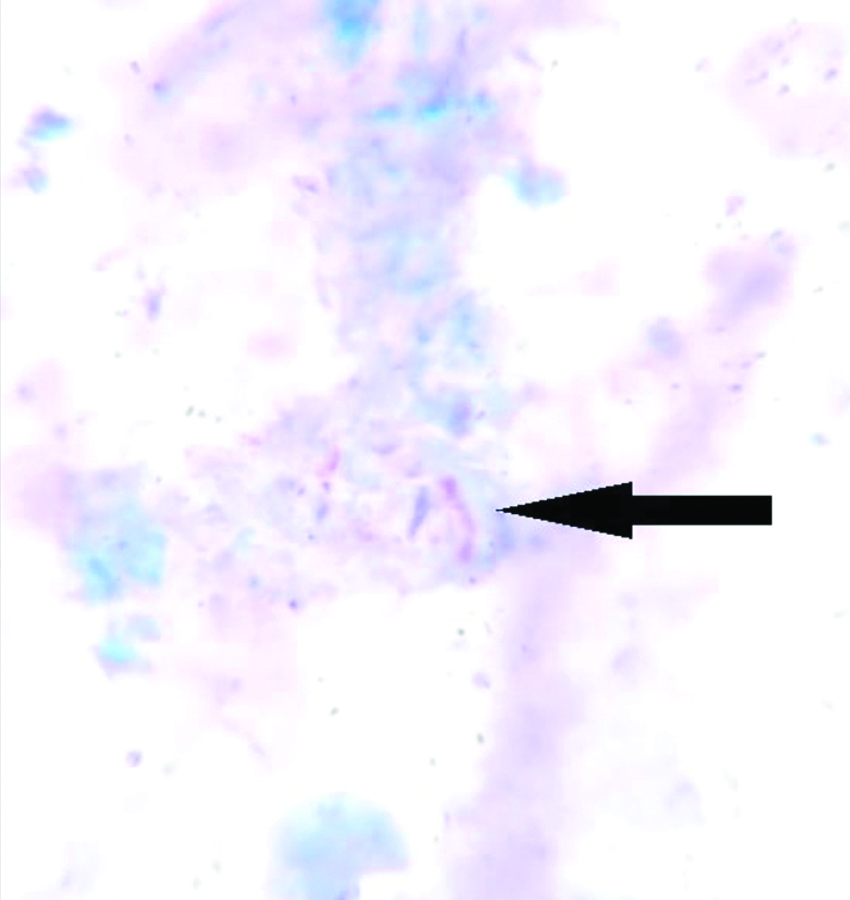
A bone marrow biopsy was performed in view of PUO, pancytopenia, and hepatosplenomegaly. Histopathological Examination (HPE) of the bone marrow biopsy revealed hypercellular marrow with epithelioid cell granulomas and the presence of AFB on the Ziehl-Neelsen (ZN) stain [Table/Fig-2], alongside marked bone marrow fibrosis. The bone marrow biopsy’s CBNAAT was negative for Mycobacterium tuberculosis. Bone marrow aspiration culture were awaited.
The image shows the presence of AFB (black arrow) in a bone marrow aspiration slide (Ziehl-Neelsen stain, 10x).
The patient was empirically started on ATT with the HRE regimen: Isoniazid (H) 5 mg/kg, Rifampicin (R) 10 mg/kg, and Ethambutol (E) 15 mg/kg while awaiting the bone marrow culture reports. The patient was discharged on the HRE regimen and instructed to follow-up regarding the bone marrow culture reports.
The patient presented to the emergency department after four weeks with acute complaints of difficulty initiating urination and pain in the hypogastric region, associated with bladder fullness. The patient also complained of stiffness in both lower limbs and buckling of the knees while walking. There were no complaints of headache, painful eye movements, or decreased vision. Upon questioning, the patient revealed that he had stopped taking ATT after one week because he experienced symptomatic relief. In the CNS examination, the patient demonstrated reduced power (4/5) in both lower limbs. Deep tendon reflexes were exaggerated (3+) in both lower limbs, and the Babinski sign was positive bilaterally. Vibration sensation and fine touch sensation were symmetrically reduced by 50% in both lower limbs, and there was a positive Romberg’s sign. The remainder of the examination was within normal limits. There were no signs of cerebellar or meningeal involvement, and no cranial nerves were affected. The fundus examination revealed normal findings, with no signs of papilloedema or optic neuritis. The CSF analysis showed lymphocytic pleocytosis, elevated CSF protein, and low CSF glucose, suggestive of Tuberculous Meningitis (TBM). However, the CSF, CBNAAT, and CSF Adenosine Deaminase (ADA) levels tested negative for TB.
Electrocardiography and chest radiography were unremarkable. Ultrasonography of the abdomen and pelvis showed hepatosplenomegaly similar to the previous report, along with a significant post-void volume of 850 cc. Laboratory investigations indicated persistent bicytopenia [Table/Fig-3].
Initial laboratory findings on second admission.
| Investigations | Values | Normal values |
|---|
| Haemoglobin (gm%) | 9 | 13.5-17.5 |
| Total leukocyte count (cells/mm3) | 5200 | 4000-11000 |
| Platelet count (/mm3) | 90000 | 150000-450000 |
| CSF R/M: | TLC: 16 (90% lymphocytes) | 0-5 cells/mm3 (lymphocytes) |
| Glucose: 28 (less than 2/3rd of corresponding blood sugars) | Equal to blood glucose levels or >45 mg/dL |
| Protein: 123 (albumin <0.3) | 15-45 mg/dL |
| CSF ADA (U/L) | 7.5 | Less than 10 |
| CSF CBNAAT | Negative | Negative |
| CSF C/S | No growth | No growth |
| CSF Indian ink stain | Negative | Negative |
| CSF Toxoplasma IgM | Negative | Negative |
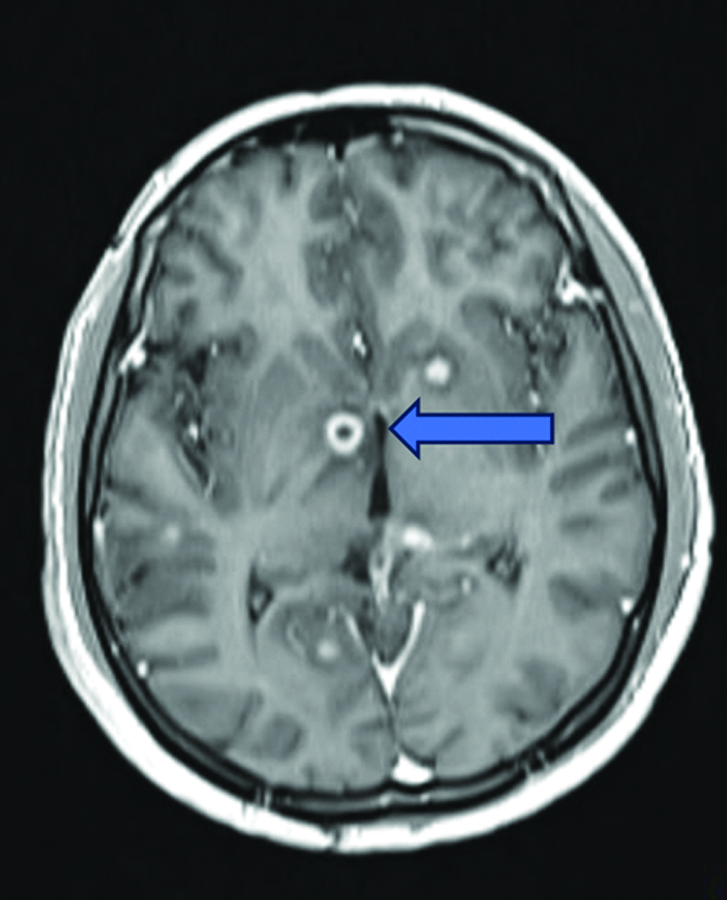
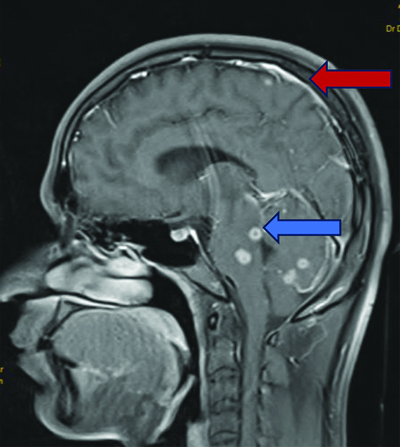
Magnetic Resonance Imaging (MRI) of the brain revealed multiple ring-enhancing lesions in the bilateral cerebral and cerebellar hemispheres, as well as the brainstem, with perilesional oedema suggestive of a granulomatous infection, most likely a tuberculoma [Table/Fig-4]. Diffuse leptomeningeal enhancement was observed, indicative of meningitis [Table/Fig-5].
MRI T1WI coronal section shows multiple ring-enhancing lesions (blue arrow) with perilesional oedema in the basal ganglia, suggestive of tuberculoma.
MRI T1WI sagittal section shows multiple ring-enhancing lesions (blue arrow) in the brainstem and cerebellum, accompanied by meningeal enhancement (red arrow).
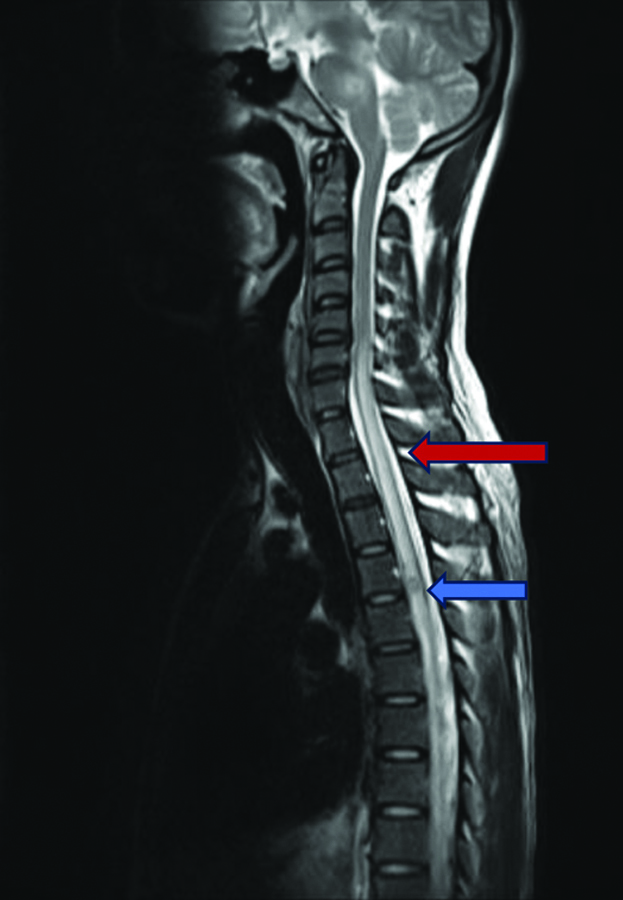
MRI of the dorsal spine showed a single well-defined round ring-enhancing lesion in the intramedullary part of the dorsal spinal cord at the D4-D5 intervertebral disc level, with an extensive long-segment intramedullary T2 hyperintense signal in the cervical and dorsal spinal cord extending from C6 to D9 vertebrae [Table/Fig-6].
MRI of the dorsal spine (sagittal section) showing extensive contiguous long segment intramedullary lesion (red arrow) which is hyperintense on T2-weighted images in the cervical and dorsal spinal cord extending from C6 to D9 vertebra. Single well-defined round ring-enhancing lesion (blue arrow) in the intramedullary part of the dorsal spinal cord at D4-D5 intervertebral disc level.
The patient was started on injection dexamethasone 8 mg three times daily, along with antitubercular therapy using the HRZE regimen, which includes isoniazid 5 mg/kg, rifampicin 10 mg/kg, pyrazinamide 20 mg/kg, and ethambutol 15 mg/kg.
After six weeks, brown, granular growth was observed in the Lowenstein-Jensen (LJ) medium [Table/Fig-7], and the Mycobacterium tuberculosis complex was detected in Polymerase Chain Reaction (PCR) with sensitivity to rifampicin and isoniazid. Thus, the final diagnosis of LETM and TB involving the bone marrow was microbiologically and radiologically confirmed.
Brown, granular growth in Lowenstein-Jensen (LJ) medium.

The patient was evaluated for an immunocompromised state due to widespread disseminated infection and underwent repeat HIV ELISA and P25 antigen tests, which were negative. A CD4 total count was conducted and found to be significantly reduced (240 cells/c.mm).
Due to financial constraints, further evaluation for idiopathic CD4 immunodeficiency was not pursued. Dexamethasone was tapered slowly over two months. At the two-month follow-up, the patient’s complaints of urinary retention and stiffness while walking had resolved. The patient completed a 12-month intensive ATT (HRZE) phase and transitioned to the continuous ATT (HRE) phase. Significant clinical improvement was observed during regular follow-ups.
Discussion
In India, a significant number of individuals suffer from both pulmonary and extrapulmonary TB. The spectrum of CNS-TB primarily encompasses conditions such as tubercular abscess, SIMT, cerebral miliary TB, tubercular arteritis, TB meningoencephalitis, and tuberculoma. SIMT is the least common type of CNS-TB and occurs even more rarely as LETM. It seldom meets the criteria for LETM. Therefore, physicians frequently consider alternative diagnosis for LETM, such as metabolic myelopathy, Myelin-Oligodendrocyte Glycoprotein-Associated Encephalomyelitis (MOG-EM), viral infections, Neuromyelitis Optica Spectrum Disorders (NMOSD), and connective tissue diseases, often overlooking the TB association of LETM, particularly in patients without primary pulmonary TB [1]. Less than 2% of all TB cases and 20% of all extrapulmonary TB cases are attributed to disseminated TB [2]. In disseminated TB, normocytic normochromic anaemia is the most common manifestation. Additionally, thrombocytopenia and neutropenia have also been observed [3]. Pancytopenia rarely manifests as a haematological abnormality in cases of disseminated TB. Patients presenting with pancytopenia should be evaluated for granulomas in the bone marrow caused by bone marrow TB rather than attributing the condition to hypersplenism or haemophagocytosis [2]. Disseminated TB occurs when Mycobacterium tuberculosis affects at least two non contiguous organs simultaneously or involves the blood or bone marrow [4]. This severe form of TB can be life-threatening and is caused by the extensive spread of the bacteria through lymphatic and haematogenous routes [4].
The primary manifestations of CNS-TB are mainly TBM (95%), followed by tuberculous abscess and cerebral tuberculoma. Less frequent presentations include tuberculous pachymeningitis, spinal arachnoiditis, and calvarial TB [5,6]. SIMT is an uncommon occurrence, with only a handful of case reports documented in the literature [7,8]. Rarely, SIMT presents as LETM, possibly due to an unclear immunological-inflammatory reaction to the infectious pathogen. The characteristics of LETM include severe neurological deficits, hyperintense lesions on T2-weighted MRI of the spinal cord spanning three or more contiguous vertebral segments, and extensive inflammation. The cervico-dorsal region of the spinal cord is commonly affected by LETM [1]. In tuberculous myelitis, CSF analysis typically reveals an ongoing inflammatory reaction characterised by predominantly lymphocytic pleocytosis and elevated protein levels [8].
In present case, the spinal lesion was new, as he had no symptoms of urinary retention or stiffness in his legs during the first admission. This could be attributed to a paradoxical response to TB treatment or the discontinuation of ATT. The paradoxical response during treatment refers to a scenario in which a patient, who had previously shown improvement with therapy, experiences a worsening of clinical symptoms or the appearance of new lesions on imaging [8,9]. The exact cause of this paradoxical response is not fully understood, but several hypotheses have been suggested. According to one theory, ATT leads to the breakdown and release of tubercular proteins, triggering a robust inflammatory reaction that can result in the development of new lesions or the enlargement of existing ones [9]. Another theory proposes that it could be an immune response to the primary tuberculous infection or the tubercular bacilli. Additionally, another potential explanation could be the ineffectiveness of ATT due to inadequate penetration into the CSF after the initial inflammation diminishes [8]. The most probable mechanism of LETM was attributed to the discontinuation of ATT.
Disseminated TB can manifest with a range of haematological abnormalities, but pancytopenia is uncommon. Several theories have been proposed to explain pancytopenia in disseminated TB, such as developmental maturation arrest of cells, hypersplenism, and histiocytic hyperplasia. Pancytopenia caused by granulomatous infiltration of the bone marrow leading to fibrosis is exceedingly rare, with an incidence ranging from 0.4 to 2% [2]. Patients diagnosed with disseminated or miliary TB, where granulomas are present in the bone marrow, typically exhibit severe normocytic normochromic anaemia, peripheral monocytopenia, and histiomonocytosis with caseating necrosis in the bone marrow [3]. TB in the bone marrow can be diagnosed by the presence of caseating necrosis and AFB within the granulomas. This condition can also lead to an increase in reticulin fibers, potentially resulting in secondary myelofibrosis [10]. In index patient, pancytopenia was caused by bone marrow infiltration by granulomas and secondary myelofibrosis due to TB. Therefore, it is crucial to maintain a high suspicion of TB involvement in the bone marrow in patients with disseminated TB presenting with pancytopenia, rather than attributing it to hypersplenism.
The diagnosis of TB is difficult because of the shortcomings of current diagnostic techniques. Confirmation typically requires the identification or culture of AFB. Unfortunately, clinical and imaging findings are non specific for TB, and laboratory techniques are often insensitive or slow. This has resulted in a delay in definitively diagnosing the patient with disseminated TB and initiating ATT for him. The microscopic examination of CSF for AFB has a sensitivity of approximately 10-20% in cases of extrapulmonary TB, particularly in cases of TBM [11]. According to a comprehensive review and meta-analysis, the specificity and sensitivity of CBNAAT were found to be 98% and 56%, respectively [12-14].
In this patient, both the bone marrow CBNAAT and the CSF CBNAAT were negative, which complicated the diagnosis of TB. However, the diagnosis was confirmed by culturing bone marrow aspiration on Löwenstein-Jensen (LJ) solid media, followed by PCR. Therefore, it is crucial to consider TB in the differential diagnosis if there is a strong clinical suspicion, despite a negative CBNAAT report, particularly in endemic countries like India.
To clinically diagnose disseminated TB, an AFB smear and culture on both liquid and solid media, as well as PCR and/or histological evidence, are required. Disseminated TB is diagnosed when a patient meets one of the following criteria: Mycobacterium tuberculosis isolation, a positive PCR result, or histological evidence of caseating granulomatous inflammation in blood, bone marrow, or in at least two non contiguous organs, with or without the presence of miliary TB. Furthermore, the diagnosis can be verified by isolating Mycobacterium tuberculosis, obtaining a positive PCR result, or histologically identifying caseating granulomas in one organ, in conjunction with radiographic evidence of miliary lung lesions [15].
This patient presented with PUO persisting for one month. A PUO is defined as a persistent fever above 38.3°C (100°F) that remains undiagnosed despite at least three weeks of investigation, including one week of hospital evaluation [16]. Empirical treatment is recommended in three distinct situations: initiating ATT for suspected CNS or miliary TB, prescribing steroid treatment for suspected temporal arteritis, and administering antimicrobial therapy for patients with suspected infective endocarditis and signs of sepsis [16]. As there was a high suspicion of TB involving the bone marrow, following positive AFB staining, empirical ATT was initiated for the patient.
Sepkowitz KA conducted a study involving 3,056 patients from 1930 to 1997. In this study, TB constituted 7.9% of all diagnosis of PUO. Among the 1,366 patients with detailed information on TB sites, pulmonary TB represented 2.3% of all cases, while extrapulmonary TB accounted for 4.8%. TB remains a significant final diagnosis for patients presenting with PUO. In cases where a thorough diagnostic examination yields inconclusive results, empirical ATT should be considered [17].
For TB-related transverse myelitis, the initial treatment regimen includes oral administration of pyrazinamide at 1.5 g/day, isoniazid at 600 mg/day, ethambutol at 15 mg/kg/day, and rifampicin at 450 mg/day for a minimum of 12 months. Additionally, a concurrent 4-week course of intravenous dexamethasone therapy was initiated, starting at 0.4 mg/kg/day in the first week and tapering to 0.1 mg/kg/day by the fourth week. This was followed by four weeks of oral dexamethasone, starting at 4 mg/day and reducing by 1 mg weekly [18]. Alternatively, high-dose intravenous methylprednisolone may offer potential benefits in the treatment of TB transverse myelitis due to its anti-inflammatory effects. Corticosteroids are beneficial in managing both TB transverse myelitis and brain tuberculoma when administered alongside ongoing ATT [8].
Conclusion(s)
This case illustrates the complexity and diagnostic challenges of disseminated TB, particularly with atypical presentations such as LETM and bone marrow involvement. Despite negative initial diagnostic tests, the presence of AFB on bone marrow biopsy and culture ultimately confirmed the diagnosis of TB. The patient’s response to ATT underscores the importance of considering TB in the differential diagnosis for patients with persistent fever, pancytopenia, and unexplained neurological symptoms, especially in TB-endemic regions. Timely initiation and adherence to ATT, alongside appropriate corticosteroid therapy, are crucial for managing disseminated TB and preventing complications. This case also highlights the need for heightened clinical suspicion and comprehensive evaluation in cases of PUO, ensuring that TB is not overlooked, even when initial tests are inconclusive.
TIBC: Total iron binding capacity; HIV: Human immunodeficiency virus; HbsAg; Hepatitis B surface antigen; HCV: Hepatitis C virus; C/S: Culture and sensitivity
[1]. Anand KA, Bhowmik KK, Sarkar A, Ghosh R, Mandal A, Swaika B, Tubercular longitudinally extensive transverse myelitis (LETM): An enigma for primary care physiciansJ Family Med Prim Care 2021 10(2):1057-60.Epub 2021 Feb 27. PMCID: PMC813834110.4103/jfmpc.jfmpc_2101_2034041124 [Google Scholar] [CrossRef] [PubMed]
[2]. Tahir M, Nida A, Qamar S, Hiding in the bone: A case of miliary tuberculosis with bone marrow involvementAME Case Rep 2018 2:36PMCID: PMC615559510.21037/acr.2018.06.0530264032 [Google Scholar] [CrossRef] [PubMed]
[3]. Singh KJ, Ahluwalia G, Sharma SK, Saxena R, Chaudhary VP, Anant M, Significance of haematological manifestations in patients with tuberculosisJ Assoc Physicians India 2001 49:788:790-94.11837465 [Google Scholar] [PubMed]
[4]. Anilkumar A, Manikandan S, Sreenivasan V, Rare presentation of disseminated tuberculosis in the present era of modern tuberculosis managementInt J Med Pharm Case Reports 2022 15(4):88-94.10.9734/IJMPCR/2022/V15I4318 [Google Scholar] [CrossRef]
[5]. Tariq R, Ahmed R, Tuberculous hypertrophic pachymeningitis presenting as visual blurring and headachesJ Pak Med Assoc 2012 62(9):966-68.23139987 [Google Scholar] [PubMed]
[6]. Naidoo DP, Desai D, Kranidiotis L, Tuberculous meningiomyeloradiculitis--A report of two casesTubercle 1991 72(1):65-69.10.1016/0041-3879(91)90026-o1882446 [Google Scholar] [CrossRef] [PubMed]
[7]. Lin SK, Wu T, Wai YY, Intramedullary spinal tuberculomas during treatment of tuberculous meningitisClin Neurol Neurosurg 1994 96(1):71-78.10.1016/0303-8467(94)90033-78187386 [Google Scholar] [CrossRef] [PubMed]
[8]. Jain RS, Kumar S, Tejwani S, A rare association of tuberculous Longitudinally Extensive Transverse Myelitis (LETM) with brain tuberculomaSpringerplus 2015 4:47610.1186/S40064-015-1232-Z [Google Scholar] [CrossRef]
[9]. Cheng VC, Ho PL, Lee RA, Chan KS, Chan KK, Woo PC, Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patientsEur J Clin Microbiol Infect Dis 2002 21(11):803-09.Epub 2002 Nov 710.1007/s10096-002-0821-212461590 [Google Scholar] [CrossRef] [PubMed]
[10]. Samuelsson SM, Killander A, Werner I, Stenkvist B, Myelofibrosis associated with tuberculous lymphadenitisActa Med Scand 1966 179:326-35.10.1111/J.0954-6820.1966.TB02379.X [Google Scholar] [CrossRef]
[11]. Diggikar PM, Mundada M, Reddy HR, Pancholi T, Yammanuru BR, Garg L, Atypical presentation of tuberculous meningitis-challenges in diagnosis: A case reportIndian J Respir Care 2024 13(1):69-72.10.5005/JP-JOURNALS-11010-1090 [Google Scholar] [CrossRef]
[12]. Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, Tuberculous meningitis: A uniform case definition for use in clinical researchLancet Infect Dis 2010 10(11):803-12.Epub 2010 Sep 610.1016/S1473-3099(10)70138-920822958 [Google Scholar] [CrossRef] [PubMed]
[13]. Ho J, Marais BJ, Gilbert GL, Ralph AP, Diagnosing tuberculous meningitis-have we made any progress?Trop Med Int Health 2013 18(6):783-93.Epub 2013 Mar 2510.1111/tmi.1209923521060 [Google Scholar] [CrossRef] [PubMed]
[14]. Thwaites GE, van Toorn R, Schoeman J, Tuberculous meningitis: More questions, still too few answersLancet Neurol 2013 12(10):999-1010.Epub 2013 Aug 2310.1016/S1474-4422(13)70168-623972913 [Google Scholar] [CrossRef] [PubMed]
[15]. Khan FY, Review of literature on disseminated tuberculosis with emphasis on the focused diagnostic workupJ Family Community Med 2019 26(2):83-91.PMCID: PMC651576410.4103/jfcm.JFCM_106_1831143078 [Google Scholar] [CrossRef] [PubMed]
[16]. Fernandez C, Beeching NJ, Pyrexia of unknown originClin Med (Lond) 2018 18(2):170-74.PMCID: PMC630344410.7861/clinmedicine.18-2-17029626024 [Google Scholar] [CrossRef] [PubMed]
[17]. Sepkowitz KA, Tuberculosis as the cause of fever of unknown origin: A reviewInt J Infect Dis 1997 2(1):47-51. [Google Scholar]
[18]. Jiang Y, Xu X, Guo Z, Liu Y, Lin J, Suo L, Myelitis: A common complication of tuberculous meningitis predicting poor outcomeFront Neurol 2022 13:830029PMCID: PMC896583310.3389/fneur.2022.83002935370906 [Google Scholar] [CrossRef] [PubMed]