Materials and Methods
This in-vitro study was designed to analyse both qualitative and quantitative analysis of the Wrightia tinctoria extracts, its effects over cell viability that gives an idea about toxicity, and their therapeutic values. The study was conducted at SRM Institute of Science and Technology, Chennai and the duration of the study was eight months. Ethical approval was obtained from the Institutional Ethical Committee Letter No. 2441/IEC/2021 dated 27/05/2021.
Collection of plant and authentication:Wrightia tinctoria was collected from the Araku valley in Eastern Ghats of Visakhapatnam, Andhra Pradesh, India. The taxonomic identification of Wrightia tinctoria was confirmed by the morphological analysis and authenticated by a qualified botanist. Unwanted dust particle from fresh plant material (leaves and stems) was removed by washing under running tap water and with distilled water then shade dried for 14 days. The shade dried plant material blended to a fine powder using the domestic blender, stored in airtight containers at 4°C until further used [8].
Preparation of extracts from Wrightia tinctoria: Dried powder of Wrightia tinctoria was subjected to solvent extractions by using a Soxhlet apparatus. Extract was prepared with three solvents (ethyl acetate, ethanol and aqueous). A thimble containing about 30 g of Wrightia tinctoria powder was placed in a Soxhlet apparatus flask with 300 mL of solvent, and extraction was carried out employing different solvents in the following order of increased polarity: ethyl acetate, ethanol and aqueous until the solvent in the separator syphon tube turned transparent. For extract filtering, Whatman No. 1 filter paper was employed. The filtered extracts were concentrated at 40°C in a vacuum oven, and then stored in a cold, dark environment until use.
Preliminary phytochemical analysis [9]: Preliminary phytochemical analysis was performed for all three solvent extracts of Wrightia tinctoria. All three extracts were qualitatively analysed for identification of various phytoconstituents such as alkaloids, saponins, quinines, tannins, glycosides, flavonoids, phenolic compounds, proteins, terpenoids and steroids by using standard protocols [Table/Fig-1] [9].
Phytochemical analysis of Wrightia tinctoria.
| S. No. | Phytochemicals | Inference |
|---|
| Ethyl acetate | Ethanol | Aqueous |
|---|
| 1. | Alkaloids | (-) | (+) | (+) |
| 2. | Saponins | (-) | (++) | (+) |
| 3. | Quinines | (-) | (-) | (-) |
| 4. | Tannins | (-) | (+++) | (+) |
| 5. | Glycosides | (-) | (++) | (+) |
| 6. | Flavinoids | (-) | (+++) | (+) |
| 7. | Phenolic compounds | (-) | (++) | (+) |
| 8. | Proteins | (-) | (+) | (+) |
| 9. | Terpenoids | (+) | (+) | (+) |
| 10. | Steroids | (+) | (+) | (+) |
The signs “-”, “+”, “++”, and “+++” are used to indicate the relative presence or abundance of specific phytochemicals in the plant extract. The indicators are qualitative in nature where the signs “-”, “+”, “++”, and “+++” represents negative, positive, moderately positive and strongly positive presence of the phytochemicals in the extract
Mayer’s test (Alkaloids Test) [9]: A 2 mL of 1% HCl was added to 5 mL of extract. The mixture was then mixed with Mayer’s reagent. Alkaloids are indicated by turbidity.
The foam test (saponin test) [9]: After mixing 5 mL of extract with 5 mL of distilled water, the mixture was heated. The presence of saponins is indicated by the production of stable foam.
Quinine testing [9]: A few drops of strong sulfuric acid were poured along the test tube’s edges to 1 mL of extract. Quinines are indicated by their red appearance.
Tannin test (a lead acetate test) [9]: A few drops of FeCl3 were added after 2 mL of the extract and 2 mL of distilled water were mixed together. Tannins are indicated by green precipitate.
Glycosides test [9]: A 1 mL of Fehling’s solution was boiled and added to 1 mL of extract. Glycosides are indicated by orange precipitate.
Ferric chloride test: a flavonoid test [9]: One millilitre of 10% lead acetate solution was added to one millilitre of extract. The presence of flavonoids is shown by the production of a yellow precipitate.
Phenolic substances [9]: A tiny amount of the extract was diluted with water. A solution of ferric chloride was used the dilute. Phenolic substances are indicated by a violet colour.
Protein test (Biuret test) [9]: Two drops of 0.1% copper sulphate and 10% sodium hydroxide were used to treat the extract. Proteins are indicated by a violet or pink tint.
Terpenoids [9]: A 5 mL of extract was mixed with 2 mL of chloroform and 3 mL of strong sulfuric acid in that order. Terpenoids are indicated by a reddish-brown interface in the solution.
Steroid usage [9]: Two millilitres of acetic anhydride and one millilitre of extract were combined with 2 mL of sulfuric acid. Steroids are present if the colour changes from violet to blue or green.
MTT assay for assessment of cytotoxicity against vero cell line
MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) -the solution is filtered through a 0.2 μm filter and kept frozen for long periods of time or at 2-8°C for frequent usage.
The DiMethylsulfoxide (DMSO).
CO2 incubator
Reader for microplates
An inverted microscope
Cooling centrifuge
Preparation of test solutions: Serial two-fold dilutions ranging from 3.125 to 100 μg were produced for the MTT test.
Culture medium and cell lines: The National Centre for Cell Sciences (NCCS), Pune provided the Vero cell line, which was cultivated in Dulbecco’s Modified Eagle Medium (DMEM) media supplemented with 10% inactivated Foetal Bovine Serum (FBS), penicillin (100 IU/mL), and streptomycin (100 μg/mL) at 37°C in a humidified atmosphere with 5% CO2 until confluent.
Procedure
Using the appropriate media containing 10% FBS, the monolayer cell culture was trypsinised and the cell count was adjusted to 1.0×105 cells/mL. A volume of 100 μL of the diluted cell suspension (1×104 cells/well) was introduced into every well of the 96-well microtiter plate. Once a partial monolayer had developed after 24 hours, the supernatant was discarded, the monolayer was once again washed with medium, and 100 μL of various test sample concentrations were applied to the partial monolayer in microtiter plates. After that, the plate was incubated for 24 hours at 37°C in an environment 5% CO2.
Following the incubation period, each well received 20 μL of MTT (2 mg/1 mL of MTT in PBS), with the test solutions in the wells being removed. The plate was incubated in a 5% CO2 environment at 37°C for four hours. After removing the supernatant, 100 μL of DMSO was added, and the plate was gently shaken to dissolve the formazan that had formed. At 570 nm, the absorbance was determined with a microplate reader. The formula used to compute the percentage of viability was % viability=Sample abs/Control abs×100 [10].
Results
The phytochemical analysis of the extract on various solvents revealed presence of many bioactive compounds as tabulated in [Table/Fig-1].
Of the three extracts ethyl acetate, ethanol and aqueous, the ethanolic extract showed higher quantifiable bioactive compounds namely the tannins, flavonoids, saponins, glycosides and phenolic compounds when compared to the other two extracts. The presence of this various bioactive compounds is responsible for the pharmacological actions against oral disorders.
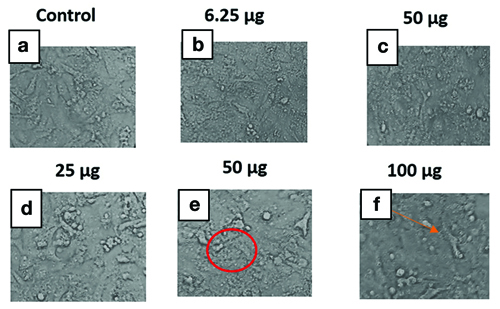
Microscopic images of Vero cells cultured with varying concentration of Wrightia tinctoria extract, showing normal cell morphology and confluence are described in [Table/Fig-2].
Effect of Wrightia tinctoria ethanolic extract at varying concentrations on Vero Cell lines. a) Represents normal vero cells; b-d) Shows no change in the morphology as compared to the of control group cells; e) Shows strong disintegration, condensation and loss in morphology showing branch like shape and elongation of the cells (Circle); f) Reveals dose dependent cytotoxic response of Wrightia tinctoria extract on vero cell line characterised, and loss of adherence (Arrow).
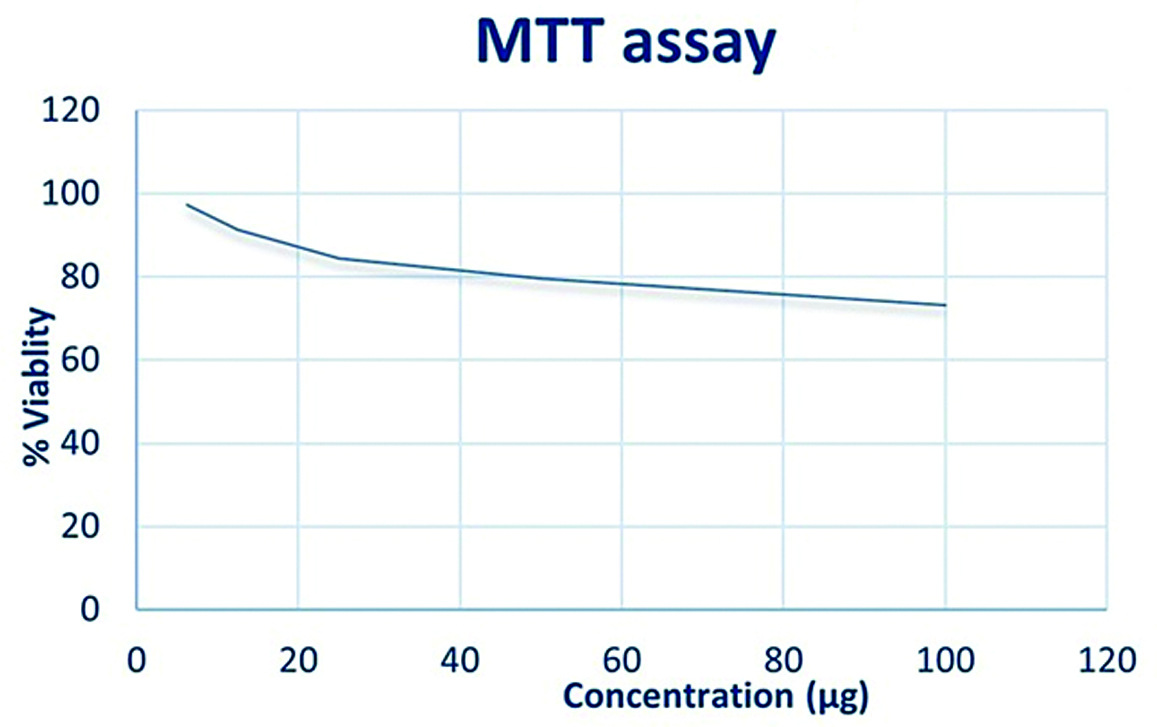
Ethanolic extract of wrightia tinctoria was used at varying concentrations. The concentration required for 50% inhibition (IC50) was calculated. The assay was performed and triplicated and the % of cell viability was calculated.
Graphs were plotted with various concentration of the ethanolic extract of Wrightia tinctoria sample on the x-axis and the percentage of viability of the Vero cells at various concentrations on the y-axis [Table/Fig-3]. With the ethanolic extract demonstrated the cytotoxicity indices IC 50 >100 which indicates even with the concentration of 100 μg/mL more than 70% of the cells were viable.
Concentration versus cell viability.
Discussion
The plant extract and the phytochemical analysis were performed in accordance previously established standard methods [11,12]. The current study observed the presence of large number of bioactive compounds in the ethanolic extracts of the Wrightia tinctoria including steroids, phenolic compounds, flavonoids, glycosides, saponins and tannins. These compounds are normally present in many regular food items and hold great potential to be used as drugs for various diseases due to their safety and less cytotoxicity which is in accordance with the study done to assess the bioactive components [13]. These phytoconstituents have been extensively studied for their diverse pharmacological activities, including antioxidant, anti-inflammatory, and anticancer properties. These properties can be utilised in treating oral conditions like oral ulcers, promotion of wound healing and various inflammatory conditions affecting the gingiva and the oral mucosa. Alkaloids, for instance, have been reported to exhibit cytotoxic effects against cancer cells by inducing apoptosis and cell cycle arrest [14]. Similarly, flavonoids have shown promising anticancer potential through various mechanisms such as inhibition of cell proliferation and angiogenesis, and induction of apoptosis [15,16].
The cytotoxicity evaluation of Wrightia tinctoria on normal Vero cell lines using the MTT assay demonstrated dose-dependent effects. While cytotoxicity against cancer cells is desirable for therapeutic purposes, it is equally crucial to ensure the safety of natural products on normal cells. The observed cytotoxic effects may be attributed to the presence of bioactive compounds in Wrightia tinctoria, which could interfere with cellular processes and viability [17,18]. The MTT assay is a commonly used method for evaluating cell viability and cytotoxicity in various biological and pharmaceutical studies [19]. The present study with the ethanolic extract demonstrated the cytotoxicity indices IC 50> 100 (half maximal Inhibitory concentration) which indicates even with the concentration of 100 μg/mL more than 70% of the cells were viable [Table/Fig-3]. This is in accordance with study done by Chaudhary S et al., where petroleum ether and ethyl acetate extracts of Wrightia tinctoria doesn’t have lethal effects on non-tumour fibroblast [20]. Assessing the cytotoxicity of Wrightia tinctoria using the MTT assay can provide valuable insights into its potential therapeutic benefits and help in understanding role in promoting overall health and wellness [21,22].
Limitation(s)
In the current study, the crude extract of the plant was used to study the cytotoxicity in the cell line; however, further studies should be conducted with specific bioactive compounds present in the plant extract as the toxic profiles of the individual biomolecules may have different effects on the cell line. Also, studies on animal models should be conducted to ensure the safety of the extract in humans.
Conclusion(s)
According to the current research, Wrightia tinctoria was a significant plant with highly potent therapeutic properties. The plant exhibits varied phytochemical elements and the results of this investigation demonstrated a noteworthy level of IC50 >100 when various concentrations of ethanolic extracts of Wrightia tinctoria leaf in normal cell line which confirms the safety of the use of the plant extract. Also, further studies pertaining to various components present in the plant extract for assessment of their potential in treating various oral diseases can be conducted done in the future.
The signs “-”, “+”, “++”, and “+++” are used to indicate the relative presence or abundance of specific phytochemicals in the plant extract. The indicators are qualitative in nature where the signs “-”, “+”, “++”, and “+++” represents negative, positive, moderately positive and strongly positive presence of the phytochemicals in the extract