Adenoid Cystic Carcinoma (ACC) is an uncommon form of malignant neoplasm of the salivary gland. ACC is a rare epithelial, slow-growing yet aggressive neoplasm with a remarkable capacity for recurrence. It most commonly develops in the salivary glands or other regions of the head and neck. Hereby, authors present a case of a 72-year-old female who presented with a rapidly enlarging swelling in the right submandibular region, which had recurred three times. Histopathological examination reported it as ACC grade II. ACC is known for its ability to invade perineurally, its high frequency of local and distant recurrence and its poor long-term prognosis. In the present case, the treatment provided was complete surgical resection. The patient underwent wide surgical excision of the lesion with appropriate margins, as well as a completion neck dissection of level IA. The histopathological diagnosis is based on tissue examination findings that exhibit typical histologic features. The presence and percentage of solid architecture reflect clinical outcomes and should be reported in the pathology report. Current diagnostics mainly rely on histomorphology, along with immunohistochemistry and cytogenetics. Poor prognostic factors include large tumour size, solid growth pattern, positive surgical margins, nodal status, nuclear atypia, high mitotic activity, high-grade transformation, lymphovascular invasion and tumour necrosis. The patient was kept on regular follow-up and there was no recurrence or metastasis noted over two years. ACC remains extremely difficult to treat due to its high recurrence and metastasis rates. The authors are presenting a case of right submandibular gland ACC for its rarity, multiple recurrences, aggressive clinical behaviour and associated radiological and histopathological findings.
Case Report
A case involving a 72-year-old female presented with rapidly enlarging and recurrent facial swelling in the right submandibular region over the past six months. She had undergone surgery twice for the ACC originating from the submandibular gland, first 7 years ago and again 1 year back.
The patient now reported increasing pain and swelling on the right-side of her face, particularly in the lateral and submandibular regions, over the last six months.
On local examination, an ill-defined, tender swelling was noted on the right-side of the face in the lower part of the submandibular region. The swelling measured 4.2×4×1.5 cm in size, was firm to hard and fixed to the underlying muscles and soft tissues of the submandibular region. The overlying skin appeared normal and there was no lymphadenopathy noted.
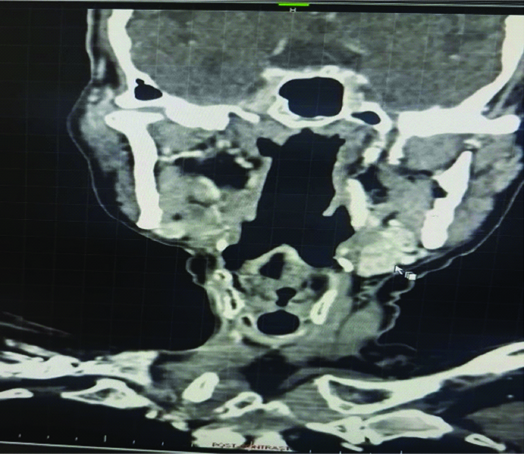
Radioimaging, including plain and contrast-enhanced scans with axial and coronal sections, revealed a heterogeneous enhancing soft-tissue mass measuring 4×3×1.5 cm in size, located in the right submandibular region on the right-side of the face. The Computed Tomography (CT) examination revealed an oval, hypodense, heterogeneous, expansively heterogeneously enhancing mass with central non enhancing areas, noted in the right submandibular region, measuring 3.8×3.6×1.6 cm [Table/Fig-1]. Anteriorly, the mass shows loss of fat planes with the mylohyoid muscle. Medially, it is seen obliterating the parapharyngeal fat. Laterally, it is limited by the mandible but shows no cortical erosion. The right sternocleidomastoid muscle is not visualised due to postoperative changes. The oral cavity is normal. The oropharynx, nasopharynx and laryngopharynx are normal. The postcricoid area appears normal and no obvious lesion is noted in the postcricoid area. The tracheal lumen appears normal. The laryngeal cartilages are normal. The hyoid bone and thyroid cartilage are normal. The thyroid gland is normal. The carotid arteries and internal jugular veins are normal. In this operated case of right submandibular ACC, a heterogenously enhancing mass with central non enhancing areas is noted in the right submandibular region as described. The oral clinical examination was normal and no significant findings were noted during the systemic examination. There was no evidence of any metastasis. The patient underwent wide surgical resection of the lesion with completion neck dissection of level IA and reverse mandibulectomy.
Computed Tomography (CT) showed oval hypodense, heterogeneous, expansive hetrogenously enhancing mass in the right submandibular region.
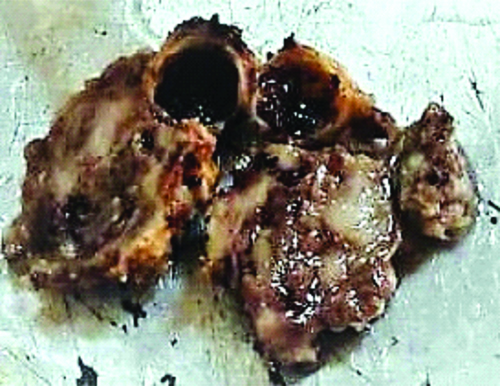
Gross examination revealed a specimen from the completion neck dissection measuring 3.8×1.6×0.4 cm. The external surface was irregular, grey-white to grey-brown and firm in consistency. The cut section showed a tumour consisting of cystic and solid areas. The solid area measured 3.2×3×1.6 cm, which was grey-white, firm, irregular and contained focal myxoid areas [Table/Fig-2]. A large cyst measuring 1 cm in diameter was noted and the cut section of this cyst shows mucoid material and blood. No lymph nodes were identified.
Ill-defined, cystic, solid swelling on the right lower part of the submandibular gland.
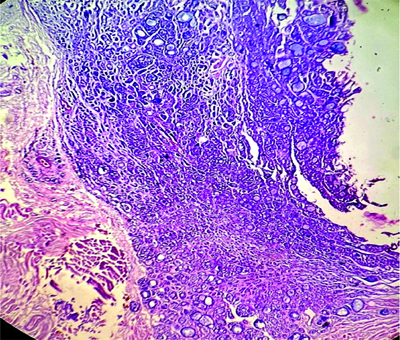
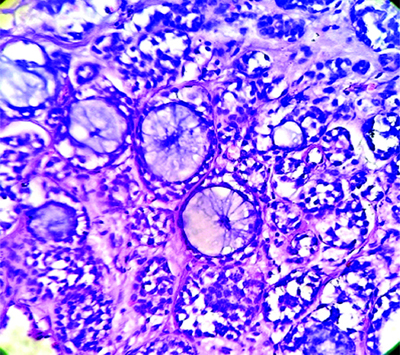
The specimen from the reverse mandible mandibulectomy consisted of a single, irregular, grey-white hard bony tissue piece measuring 4×1×0.6 cm. Microscopy showed a tumour composed of cystic and solid areas. The neoplastic cells were arranged predominantly in cribriform, tubular, glandular and solid patterns. The biphasic tumour was composed of ductal and myoepithelial cells. The cribriform pattern was predominantly composed of myoepithelial cells, which exhibited mild enlargement, pleomorphism and scant amounts of basophilic cytoplasm. In areas with a solid pattern, nests of basaloid cells were noted. The solid growth pattern constituted less than 30% of the tumour. The tubular pattern showed tubules lined by round to oval neoplastic cells exhibiting mild hyperchromasia and atypia. A few of the tubules were cystically dilated, with the lumen filled with basophilic material. The stroma showed focal areas of myxoid change, inflammation and hyalinised or myxoid globules. Also noted was a cystic area lined by cuboidal epithelium, with the wall displaying cribriform and tubular neoplastic cell patterns [Table/Fig-3,4 and 5]. There were areas showing perineural and muscle invasion.
Right submandibular gland mass, showing nodular, tubular, cribriform and cystic pattern- Adenoid Cystic Carcinoma (ACC). (H&E stain 40x).
Biphasic tumour is composed of ductal and myoepithelial cells. proliferation of uniform basaloid cells in atubular, cribriform, and solid pattern- Adenoid Cystic Carcinoma (ACC). (H&E 40x).
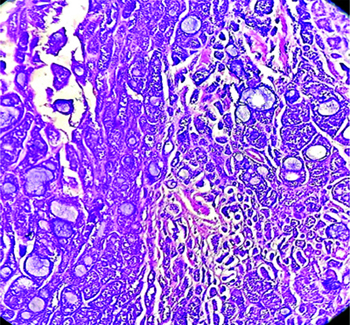
A mixture of cribriform, tubular and solid growth patterns, with solid growth pattern. (H&E 100x).
Impression: ACC grade II-right submandibular gland from completion neck dissection of the right submandibular gland. The patient underwent wide surgical resection of the lesion with appropriate margins and completion neck dissection of level IA. The patient has been kept on regular follow-up and there has been no recurrence or metastasis noted in the last two years.
Discussion
The ACC accounts for 1% to 2% of all head and neck malignancies and approximately 10% of all salivary gland neoplasms [1,2]. In the present case, the patient presented with a rapidly enlarging and recurrent swelling in the right submandibular region. ACC can arise in any salivary gland site, with approximately 50-60% developing within the minor salivary glands. It is rare in the parotid gland. Other sites include glands in the sinonasal tract, nasopharynx, oropharynx, trachea, breast, skin, cervix and prostate. These tumours present with a wide age distribution but are most common in the 5th and 6th decades of life. The incidence among females is higher than in males, with a F:M ratio of 1.2:1. Clinically, these tumours typically present as small, slowly growing mass lesions. They have the ability to invade adjacent soft tissue and even bone. This tumour has a propensity for perineural invasion (neurotropism), which is responsible for the associated pain. Cervical lymph node metastasis occurs in about 20% of cases with submandibular tumours [3].
Histopathologically, ACC shows a biphasic tumour with ductal and myoepithelial differentiation. It exhibits various patterns, including tubular, cribriform, trabecular, nests and solid [4]. The cribriform pattern is noted most commonly and is composed predominantly of myoepithelial cells with myxoid or hyalinised globules. Myoepithelial cells have dark, angulated nuclei and scant cytoplasm, giving them a basaloid appearance. The tubular pattern contains simple tubules composed of inner ductal and outer myoepithelial cells. ACC has been histologically graded according to Szanto PA et al., [5]:
Grade I: The tumour consists only of cribriform and tubular histomorphology.
Grade II: A mixture of cribriform, tubular and solid growth patterns, with the solid growth pattern comprising less than 30% of the tumour.
Grade III: Tumours with predominantly solid features (>30% of the tumour).
In a few cases, ACC shows transformation to high-grade, characterised by tumour necrosis, frequent mitosis and marked nuclear atypia.
Other rare features may occasionally present. Independent prognostic predictors include American Joint Committee on Cancer (AJCC) Tumour, Node, Metastasis (TNM) staging, margin status, high mitotic index (defined as ≥5 per 10 high power fields) and solid architecture [6].
In present case, the tumour exhibited aggressive behaviour later on, leading to recurrence. The tumour was invading the soft tissue and diffusely infiltrating the muscle. De Morais EF et al., observed that tumours with perineural invasion, positive surgical margins, cervical lymph node metastasis, solid histological features and distant metastasis are associated with increased local recurrence, a high metastatic rate and higher mortality [7]. In present case, the solid and cribriform patterns were predominant, while the ductal or tubular patterns were less prominent. The study indicated that the solid pattern is particularly associated with these aggressive features.
Newer imaging techniques, such as CT and magnetic resonance imaging, are used to determine tumour size, margins, extension, infiltration and evidence of metastasis. Histopathological diagnosis remains the standard for evaluating these cases. Immunohistochemical markers, such as CD43, may also be assessed. The genomic hallmark of ACC is a recurrent t(6;9)(q23;p23) translocation, which results in a fusion between the Myeloblastosis (MYB) and Nuclear factor I/B (NFIB) genes. This fusion protein is found in up to 90-95% of ACC tumours. Additionally, mutations in the Neurogenic Locus Notch Homolog Protein 1 (NOTCH1) gene are associated with ACC, particularly in patients with recurrent or metastatic disease [8].
The differential diagnosis includes polymorphous adenocarcinoma, basal cell adenocarcinoma and epithelial myoepithelial carcinoma [8]. These tumours typically exhibit a biphasic type with trabecular, tubular and solid growth but generally lack a cribriform growth pattern. Papillocystic architecture may be seen in epithelial myoepithelial carcinoma, while basal cell adenocarcinoma shows peripheral palisading and may include squamoid eddies. In polymorphous adenocarcinoma, the tumour exhibits cytologic monotony with only one type of tumour cell and the nuclei contain open chromatin.
Metastases commonly occur in regional lymph nodes or can spread to distant sites, such as the lungs and liver. In present case, there was no evidence of any regional or distant metastasis. The management of ACC requires a multidisciplinary approach. The primary treatment for ACC is complete surgical resection with appropriate margins [9]. Some cases may require adjuvant radiation therapy, while patients with distant metastasis may need chemotherapy. Various types of radiation may be employed depending on the cancer type, location, stage, grade and prior treatments [10].
The ACC remains an extremely challenging disease to treat due to its high rates of recurrence and metastasis. Despite aggressive therapy, locoregional recurrence occurs at rates ranging from 15% to 85% [11]. The overall survival rate for ACC is favourable, with 5-year survival rates ranging between 64% and 89% and 10-year survival rates between 37% and 77%, as noted in various studies.
Conclusion(s)
The ACC is an uncommon form of malignant neoplasm. This case presents ACC due to its aggressive clinical nature, multiple recurrences and associated radiological and histopathological findings. ACC has the ability to invade perineural tissues, a high rate of local recurrence and a poor prognosis. Long-term follow-up is advised for better management of these cases.
[1]. Nightingal J, Lum B, Ladwa R, Simpson F, Panizza B, Adenoid cystic carcinoma: A review of clinical features, treatment targets and advances in improving the immune response to monoclonal antibody therapyBiochim Biophys Acta Rev Cancer 2021 1875(2):188523 [Google Scholar]
[2]. Bradley PJ, Frequency and histopathology by site, major pathologies, symptoms and signs of salivary gland neoplasmsAdv Otorhinolaryngol 2016 78:09-16. [Google Scholar]
[3]. Zhou M, Ma T, Wang X, Zhang S, Yang G, Song R, High-risk subtype: Clinical manifestations and molecular characteristics of submandibular gland adenoid cystic carcinomaFront Oncol 2022 16:1021169 [Google Scholar]
[4]. World Health Organization Classification of TumoursPathology and Genetics: Head and Neck Tumours 2005 LyonIARC Press:221-22. [Google Scholar]
[5]. Szanto PA, Luna MA, Tortoledo ME, White RA, Histologic grading of adenoid cystic carcinoma of the salivary glandsCancer 1984 54(6):1062-69. [Google Scholar]
[6]. Xu B, Drill E, Ho A, Ho A, Dunn L, Prieto-Granada CN, Predictors of outcome in adenoid cystic carcinoma of salivary glands: A clinicopathologic study with correlation between MYB fusion and protein expressionAm J Surg Pathol 2017 41(10):1422-32. [Google Scholar]
[7]. de Morais EF, de Farias Morais HG, de Almeida Freitas R, Coletta RD, Prognostic significance of histopathological parameters for salivary gland adenoid cystic carcinomaDent J (Basel) 2023 11(11):262 [Google Scholar]
[8]. Jagtap SV, Chougule PG, Nikumbh DB, Kanetkar SR, Jain G, Polymorphous low grade adenocarcinoma presenting as recurrent oral ulcerationsJ Clin Diagn Res 2012 6(4):712-14. [Google Scholar]
[9]. Coca-Pelaz A, Rodrigo JP, Bradley PJ, Vander Poorten V, Triantafyllou A, Hunt JL, Adenoid cystic carcinoma of the head and neck: An updateOral Oncol 2015 51(7):652-61. [Google Scholar]
[10]. Warner KA, Nor F, Acasigua GA, Martins MD, Zhang Z, McLean SA, Targeting MDM2 for treatment of adenoid cystic carcinomaClin Cancer Res 2016 22(14):3550-59. [Google Scholar]
[11]. Lorini L, Ardighieri L, Bozzola A, Romani C, Bignotti E, Buglione M, Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinomaOral Oncol 2021 115:105213 [Google Scholar]